Dietary supplement enriched in antioxidants and omega-3 on human retinal pigment epithelium cells line: Evidence of protective effect against oxidative stress
Research Article - (2024) Volume 0, Issue 0
Received: 19-Jun-2024, Manuscript No. CNHD-24-139376; Editor assigned: 21-Jun-2024, Pre QC No. CNHD-24-139376 (PQ); Reviewed: 05-Jul-2024, QC No. CNHD-24-139376; Revised: 11-Jul-2024, Manuscript No. CNHD-24-139376(R); Published: 18-Jul-2024, DOI: 10.12873/0211- 6057.44. S1.006.
Abstract
Purpose: Dietary supplement enriched with antioxidants and fish oil had been shown to protect the retina from oxidative stress in vivo from light-induced retinal damage as well as Müller cells in vitro from H2O2 treatment. The objective of the present study was to evaluate the effect of this complex supplement on human retinal pigment epithelium cells line (Arising Retinal Pigment Epithelia- ARPE-19) since RPE (Retinal Pigment Epithelia) is key for nutrients uptake from the choriocapillaris and to the photoreceptors of neural retina.
Methods: We used ARPE-19 cells treated for 7 days with the dietary supplement at 11 or 44 μM eq. DHA.
Results: Herein, we demonstrated that incubation of ARPE-19 cells for 7 days with the supplement significantly reduced cells death induced by H2O2-oxidative stress from 11 μM and 44 μM equivalent DHA. Interestingly, we showed different mechanism of protection at both concentrations with a direct signal effect at 11 μM and through membrane composition modification at 44 μM. Surprisingly, at 44 μM equivalent DHA, the supplement induces phosphatidylserine externalization, an increase of isoprostanes production and caspase-3/7 activation.
Conclusion: At this high concentration, pre-conditioning effect seems to take place. Nevertheless, these results raised up the question of the long-term treatment in human.
Keywords
Eye, Fish oil, Oxidative-stress, Apoptosis, Phosphatidylserine, Plasmalogen, Fatty acids/ Oxidation, Isoprostanes, ARPE-19 cells
Introduction
Age-related Macular Degeneration (AMD) is the leading cause of blindness and visual impairment after 60 years of age in western populations[1,2]. AMD is characterized by degenerative changes within the macula, which is the central area of the retina responsible for detached vision and colour perception. The early stage of AMD is associated with an accumulation of small focal deposits called drusen under the Retinal Pigment Epithelium (RPE) cells. As the disease progresses, large RPE cell loss in focal areas can occur referred as geographic atrophy which leads to a progressive worsening of central vision. This can be followed by neovascular response arising under the retina disrupting normal retinal anatomy, known as neovascular AMD[3-7]. Although vascular endothelial growth factor inhibitors injected directly into the vitreous humour of the eye can stabilise vision in neovascular AMD, there is no treatment available for geographic atrophy. In the absence of a cure, research has focused on preventing, notably through nutritional intakes, or slowing the progression of AMD.
Several epidemiological studies (observational or interventional) have reported the benefit of an increased consumption of antioxidant and omega-3 Long Chain Polyunsaturated Fatty Acids (LC-PUFAs), including Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA), in the prevention of AMD. Supplementations with vitamins, trace elements, omega-3 fatty acids and other nutrients based on their antioxidant properties have become standard in clinical care[8]. As a consequence, supplement to improve vision have exploded on the market. However, little is known on the mechanism involved in the effect of complex supplements. Interestingly, we had previously shown that a dietary supplement enriched with antioxidants and fish oil, commercialized for clinical care of AMD patients, is associated to a protective effect against oxidative stress through modification of glutamate- glutamine cycle in vitro on Müller cells culture[9] and through changes in retinal fatty acid composition in vivo in a light-induced retinal degeneration model in rat[10]. In this in vivo experiment, the supplement was given daily by oral administration suggesting that nutrients had to go through RPE cells to reach the retina. RPE cells are involved in the uptake of nutrients from the choriocapillaris and their transfer to the photoreceptors of neural retina and in the elimination of waste from the photoreceptors[11]. Furthermore, RPE cells are also involved in visual pigment regeneration and photoreceptor outer segment renewal, then participating to maintenance of photoreceptor integrity[12]. Therefore, RPE cells are essential for photoreceptor cells survival[13]. In addition, a growing body of clinical and experimental data strongly implicate RPE cells death induced by oxidative-stress in AMD pathogenesis[14,15].
In order to go further in the mechanisms involved in this complex supplement protection, we have evaluated for the first time the effect of a dietary supplement enriched in antioxidants and omega-3 on RPE cells.
Materials and Methods
For our investigation, we used the ARPE-19 cells, a spontaneously transformed human RPE cells that conserve many biological and functional RPE cell properties, generously provided by Dunn et al. (INM, Montpellier, France)[16]. Cells were maintained in a 1:1 mixture of Dulbecco’s Modified Eagle’s Medium and Ham’s F12 medium (DMEM/ F12) supplemented with 10% foetal bovine serum and 1% penicillin-streptomycin and incubated at 37 °C in a 5% CO2 incubator for use in the subsequent experiments.
ARPE-19 cells were pre-treated with Dietary Supplement (DS) as shown in Table 1 at several concentrations (0.7 μM, 1.40 μM, 2.8 μM, 5.5 μM, 11 μM, 22 μM, 44 μM, 88 μM, 118 μM, 147 μM, 177 μM eq. DHA prepared in DMSO 0.1%) expressed as equivalent of Docosahexaenoic Acid (DHA) concentration, for 7 days. It is important to note that in our previous in vivo and in vitro experiments, the supplement was used at 11 μM eq. DHA for 5 to 7 days of treatment corresponding to the initial commercialized concentration of 365 mM[9,10]. The control group was treated with 0.1% DMSO in growth media.
| Dietary Supplement composition (DS) | Commercial mixture concentration |
|---|---|
| Ingredients | (mg/ml) |
| Vitamin and trace elements | |
| Vitamin C | 150 |
| Vitamin E | 25 |
| Zinc (sulfate) | 12.5 |
| Copper (sulfate) | 0.83 |
| Essentials fatty acids | |
| Fish oil | 580 |
| with 70% omega-3 | 405.83 |
| EPA | 231.66 |
| DHA | 115.83 |
| DPA | 14.56 |
| Extract of Tagetes erecta | |
| Lutein | 8.33 |
| Zeaxanthin | 1.66 |
| Extract of Vitis vinifera | |
| Resveratrol | 0.83 |
To induce oxidative stress on ARPE-19 cells, hydrogen peroxide was widely used in the literature[17-20]. In our experimental design, after 7 days with or without DS, oxidative stress was induced during 2 hours using 600 μM of Hydrogen Peroxide (H2O2) extemporaneously prepared by dissolving 3% H2O2 in a serum-free DMEM/F12 medium. This concentration was shown to induce 40%-50% of viability loss (unpublished data).
In our experiments, cell viability and cell death were quantified by MTT 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide assay (Thermofisher) according to manufacturer’s instructions and flow cytometry (Cytometer EPICS XL Beckman Coulter, Fullerton, CA) using annexin V-Fluorescein Isothiocyanate (V-FITC) and Propidium Iodide (PI) labelling. Viable cells are annexin V-/IP-, cells in early stage of apoptosis are annexin V+/ IP-and necrotic cells are annexin V+/IP+. The results are expressed as the percentage of total cells. To go further in apoptosis mechanisms, we evaluated caspases-3/7 activation using the ApoTox-Glo Triplex assay (Promega, France) following manufacturer’s protocol[21,22]. Moreover, cell survival assay, the clonogenic test, was performed as previously described by Villalpando and collaborators[23]. In addition, transcriptomic approach was performed as previously described[9].
Data analysis was using GraphPad Prism 7.00 and all results are expressed as mean ± SEM (Standard Error of the Mean). Statistical comparisons among groups were conducted using one-Way ANOVA. If ANOVA was significant, multiple comparisons were done to determine which pairs of mean values were different. Significant differences between groups were assessed with the post hoc Newman-Keuls test. The significance level was set at p=0.05: One symbol for p<0.05, two symbols for p<0.01, three symbols for p<0.001, and four symbols for p<0.0001.
Results and Discussion
Firstly, we evaluated the toxicity of the DS on ARPE-19 cells. Concentrations between 0.7 μM and 88 μM eq. DHA had no effect on cell viability compared to non-supplemented cells as shown in Figure 1a, green bars whereas DS at 118 μM, 147 μM and 177 μM eq. DHA induced a 41%-53% and 86% decrease in cell viability, respectively. These data show that under our experimental conditions, DS present a dose-dependent toxicity at a concentration over 88 μM eq. DHA as shown in Figure 1a, green bars. Based on this profile, we tested the potential protective effect of Dietary Supplement (DS) against oxidative stress induced by H2O2 as shown in Figure 1a, blue bars. In non-supplemented cells (-DS), H2O2 at 600 μM induced a 42% decrease (p<0.0001) in cell viability compared to no- H2O2 cells as shown in Figure 1a, blue bars as described previously[17,18]. Overall, we observed a dose dependent protective effect on cell viability of DS from 0.7 to 44 μM eq. DHA against H2O2 as shown in Figure 1a, blue bars. Nevertheless, DS from 0.7 to 2.8 μM eq. DHA have no significant effect against H2O2 as shown in Figure 1a, blue bars, whereas DS from 5.5 μM to 88 μM eq. DHA offered more than 50% protection against H2O2 as shown in Figure 1b, with full protection at 44 μM eq. DHA as shown in Figure 1a, blue bars; Figure 1b. Indeed, no more significant difference was observed between 44 μM eq. DHA treated cells with or without H2O2 as shown in Figures 1a and 1b. Over 88 μM eq.DHA, as expected since DS at these concentrations is toxic by itself, DS have no longer protection against H2O2-toxicity as shown in Figures 1a and 1b. Therefore, pre-treatment with DS has a protective effect against oxidative stress.
Figure 1a. Cell viability. Green bars represent ARPE-19 cells incubated with DS at different concentrations (from 0.7 μM to
177 μM eq. DHA) for 7 days. The results are expressed as the percentage of control condition (0 μM eq.t DHA=100% of cell
viability). Blue bars represent ARPE-19 cells pre-treated with different concentrations of DS and then exposed to 600 μM
H2O2 for 2 h. The cell viability was determined MTT assay. The results are expressed as the percentage of control condition
(without DS treatment (0 μM) or H2O2 exposure=100% of cell viability). The data are presented as means ± SEM (n=3
independent experiments, each n for each condition represented a triplicate). Significance ($) compared to 0 μM éq-DHA.
Significance (*) compared to 0 μM eq. DHA cells treated with H2O2

Figure 1c. ARPE-19 cells in flow cytometry. Cells were collected and stained with Annexin V-FITC. The results are expressed
as the percentage +of Annexin V-FITC-stained cells. The data are presented as means ± SEM (n=3 independent experiments).
Significance compared (*) to untreated cells (0 μM eq. DHA) 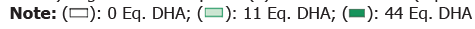
Figure 1f. 15-F2t-IsoP (isoprostanes) level was analysed by gas chromatography. The results are expressed in pg of
15-F2tIsoP in five pooled wells. The data are presented as means ± SEM (n=2 independent experiments). (H) ARPE-19 cells
were treated with two concentrations of DS (11 μM and 44 μM eq. DHA) for 7 days. Fatty acid composition was analysed by
gas chromatography. The data are presented as means ± SEM (n=2 independent experiments). Overall significance (*): One
symbol p<0.05; two symbols p<0.01; three symbols p<0.001
To better characterize the beneficial effect of the pre-treatment with DS, we evaluated its cellular effect on ARPE-19 cells at several levels without oxidative stress. Based on these results and to better characterise the effect of DS on ARPE-19 cell death or viability, we selected two DS concentrations: One of the first significant protective concentration (11 μM eq. DHA) and the most protective concentration (44 μM eq. DHA). The choice of the lower concentration (11 μM eq. DHA) is reinforced by previous data, since DS at 11 μM eq. DHA had been shown to protect in vitro Müller cells in culture against oxidative stress induced by H2O2 [24] and in vivo against light-induced retinal degeneration[10]. At 11 μM eq. DHA, no evidence of apoptosis (early or late) was observed in flow cytometry as shown in Figure 1c or caspase activation assay as shown in Figure 1d and Table 2. Unexpectedly, cells pre-treated at 44 μM eq. DHA presented both early hallmark of apoptosis[25,26] characterized by the Phosphatidylserine (PS) externalization as observed via the significant (p<0.0001) 20% increase of Annexin V+/ IP (Propidium Iodide), and late hallmarks of apoptosis as observed by the significant higher (p=0.0118) basal level of caspase-3/7 activity compared to non-treated cells (0 μM eq. DHA) as shown in Figure 1d, full bars. Nevertheless, addition of Staurosporine (S), a pro-apoptotic agent, in medium culture of cell treated with 44 μM eq. DHA cells (S44) increased the caspase activity to a similar level as untreated cells with Staurosporine (S0) as shown in Figure 1d. There is no additive effect of S (Staurosporine) on the upregulated basal activity of caspase at 44 μM eq. DHA treatment. Consequently, 44 μM eq. DHA treatment impacted only the basal level of caspase-3/7 activity and not cell viability since cells kept their proliferation ability as confirmed by the clonogenic test[23] as shown in Figure 1e. However, although no evidence of apoptosis (early or late) was observed at 11 μM eq. DHA Staurosporine (11), addition of Staurosporine, induced caspase activation Staurosporine 11 (S11) but at a significant lower level compared to untreated Staurosporine (S0) or 44 μM eq. DHA (S44) treatment. These observations are suggesting that 1/only 11 μM eq. DHA condition induced a protection against apoptosis induction by lowering caspase activation; and 2/7 days of pretreatment with 44 μM eq. DHA DS increased the apoptotic basal level apoptosis without effect on the cell response to apoptotic stress inducer.
Since retinal pre-conditioning and modification of cell response to oxidative stress could find its origin in the fatty acid composition of membranes[27], we determine the fatty acid composition of supplemented ARPE-19 cells. PS externalization might be emphasized by the change in fatty acid composition in supplemented ARPE- 19 cells, especially by the 65% increase (p<0.0001) of DHA when cells were incubated with 44 μm eq. DHA as shown in Table 2. High levels of DHA may lead to general membrane instability resulting in enhanced flip-flop, increasing the likelihood of moving PS to outer leaflet[28-32]. Moreover, when cells were incubated with 44 μm eq. DHA, the level of Arachidonic Acid (AA, C20:4 n-6) is significantly decreased by 23.6% (p<0.0001). This decrease could be explained by its use to synthesize isoprostanes. Indeed, the Isoprostanes (IsoPs) are a unique series of prostaglandin-like compounds formed via a nonenzymatic mechanism involving the free radical-initiated peroxidation of Arachidonic Acid (AA, C20:4 n-6)[33]. Among them, the 15-F2t-Isoprostanes (15-F2t-Isop) is considered as a gold standard biomarker of oxidative stress[27,34-36]. In our experimental condition, 11 μM eq. DHA had no effect on 15-F2t-IsoP production whereas 44 μM eq. DHA increased (p=0.001) 15-F2t-Isop level by 360% compared to untreated cells as shown in Figure 1f with the simultaneous, decrease (p<0.0001) by 23.6% in AA (C20:4 n-6) level. Interestingly, an increase (p<0.0001) by 202% in EPA (20:5n-3) was observed. These concomitant changes could participate to the protective effect by increasing (p<0.0001) the ratio EPA/AA by 106% as shown in Tables 2 and 3. EPA, an n-3 PUFA, is a potent antioxidant and anti-inflammatory agent and regulates the expression of various cytoprotective antioxidant enzymes[37,38]. In addition, EPA and AA compete for membrane incorporation leading to changes in membrane ratio n-3/n-6 total Polyunsaturated Fatty Acids (PUFA), thereby modulating various signalling pathways[ 39]. High n-3/n-6 ratio is key for redox homeostasis in the body[40,41]. Herein, although PUFA content was not significantly modified in 11 μM eq. DHA treated cells, total PUFA was significantly increased (<0.0001) by 38% in 44 μM eq. DHA treated cells compared to control mainly due to a decreased level of n-6 (-5.6%, <0.0001) and increased level of n-3 (+107.0%, <0.0001) leading to significant increase of n-3/n-6 ratio (+944%, <0.0001). Among n-3, the most striking increased was observed for the precursor of the α-Linolenic Acid (ALA, C18:3 n-3, +40.2%, <0.0001), n-3 Docosapentaenoic Acid (DPA, C22:5n-3, +61.4%, <0.0001) and DHA (C22: 6n-3, +65.2%, <0.0001). Regarding n-6, 44 μM eq. DHA induced an increase of Linoleic Acid (LA, C18:2 n-6, 88.2%, <0.0001), Dihomo-γ-Linoleic Acid (DGLA, C20:3 n 6; +46.7%, <0.0001) and a decrease of Docosatetraenoic (DTA, C22:4 n-6; -38.86%, <0.0001) and Arachidonic Acid (AA, C20:4 n-6; -23.6, <0.0001). Obviously, DS has an important impact on DHA pathway in the membrane. DHA is known to generate docosanoids such as Neuroprotectin D1 (NPD1) which elicits neuroprotective activity in brain ischemia-reperfusion, in oxidative-stressed retinal pigment epithelial cells; And promotes neuronal and glial cell survival[42,43]. Therefore, the observed increase in DHA precursor might participate to the neuroprotective mechanism by facilitating oxidized-DHA replacement inside the membrane[44]. In addition, Plasmalogen (Pls), a group of phospholipids with a vinyl-ether bond in the sn-1 position of glycerol[45] being sensitive markers of oxidative stress[46,47], are clearly alerted in presence of DS. Indeed, Pls level determined as the relative proportions of Dimethylacetals (DMAs) were reduced (p<0.0001) by 8.5% in cells pre-treated with 44 μM eq. DHA suggesting an increase in oxidative stress which is in accordance with our current data as shown in Figure 1. All together, these data suggest that the cellular effect of 44 μM eq. DHA, believed to be a “pre-conditioning effect”, passed through the modification of cell lipid composition. This observation is in accordance with the literature since it had been shown that the omega-3 fatty acid DHA and its derivatives (i.e. 17-HDHA, NPD1) facilitate cell survival and adaptation to stress in both in vitro and in vivo models of retinal pre-conditioning[48]. These results are also in accordance with our previous in vivo study showing that a seven-day supplementation was leading to lipid composition modification (especially DHA, EPA and AA) allowing protection to retinal degeneration 10]. Therefore, high concentrations of DHA would act like a “pre-conditioning” via lipid composition changes to ensure protection from a further stress. At the contrary, lower DHA concentrations seem to have a direct effect on cell signalling. Indeed, it appears that the state of the cells before H2O2-induced oxidative stress was different depending on the concentration, suggesting two different protective mechanisms thereafter. Indeed, with 11 μM eq. DHA treatment, no variation of lipid composition could be associated with the protection against H2O2. In this case, we could suggest that the supplement have a direct cellular signalling effect through the PUFAs it contains as it had been observed in vitro in Müller cells[9]. Interestingly, transcriptomic investigation at 11 μM eq. DHA showed few modifications of mRNA expression with 42 genes significantly deregulated as shown in Table 3. In accordance with the protective effect observed, this gene list was significantly enriched in genes involved in stress response as observed in the clustering data (i.e GO:00021666, GO:0009410, GO:0042542) suggesting a direct signalling effect of DS as shown in Figure 2.
Figure 2. Gene Ontology (GO) terms significantly enriched among differentially expressed genes in ARPE-19 cell treated
with DS. Red bars indicate the fold enrichment of the Gene Ontology (GO) and blue bars indicate the gene count. Four enriched
clusters respectively enriched with genes involved in response to stress metabolism were detected 
| 0 µM Eq. DHA | 5.5 µM Eq. DHA | 44 µM Eq.DHA | 5,5 vs. 44 Eq. DHA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Stat vs. 0 µM Eq. DHA | Mean | SEM | Stat vs. 0 µM Eq. DHA | Stat | |
| SFA 14:0 15:0 16:0 17:0 18:0 20:0 22:0 |
42,77 0,790 0,383 21,328 0,575 18,610 0,575 0,508 |
0,867 0,063 0,008 0,632 0,082 0,265 0,013 0,017 |
39,763 0,733 0,320 19,903 0,473 17,338 0,500 0,498 |
0,167 0,083 0,016 0,337 0,165 0,360 0,030 0,017 |
NS NS NS NS NS NS NS NS |
37,830 0,555 0,258 18,308 0,530 17,085 0,565 0,530 |
0,252 0,032 0,005 0,246 0,170 0,104 0,021 0,015 |
<0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 |
NS NS NS NS NS NS NS NS |
| MUFA 18:1t 16:1n-7 18:1n-7 16:1n-9 18:1n-9 20:1n-9 22:1n-9 24:1n-9 |
27,253 0,893 1,475 3,835 1,665 18,485 0,275 0,358 0,268 |
0,230 0,063 0,030 0,065 0,101 0,247 0,009 0,025 0,008 |
28,160 0,830 1,568 3,800 1,823 19,265 0,325 0,288 0,263 |
0,131 0,037 0,036 0,046 0,049 0,151 0,009 0,017 0,008 |
NS NS NS NS NS NS NS NS NS |
24,375 0,893 1,220 3,110 1,273 16,880 0,508 0,263 0,230 |
0,090 0,103 0,012 0,013 0,021 0,133 0,015 0,010 0,000 |
0,0039 NS 0,0039 0,0039 0,0039 0,0039 0,0039 0,0039 0,0039 |
<0,0001 NS <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 |
| PUFA 20:3n-9 |
23,150 0,273 |
0,307 0,028 |
25,245 0,243 |
0,196 0,011 |
NS NS |
31,943 0,323 |
0,340 0,146 |
<0,0001 <0,0001 |
0,0081 0,0081 |
| omega-6 18:2n-6 20:3n-6 20:4n-6 22:4n-6 omega-3 18:3n-3 20:5n-3 22:5n-3 22:6n-3 omega-3/omega6 |
14,093 1,838 0,948 10,298 1,010 8,853 0,328 2,815 2,075 3,635 0,628 |
0,215 0,110 0,016 0,161 0,016 0,097 0,014 0,043 0,029 0,038 0,008 |
14,813 2,405 1,108 10,450 0,850 10,190 0,303 3,725 2,145 4,018 4,523 |
0,109 0,030 0,017 0,104 0,018 0,100 0,011 0,066 0,048 0,077 0,011 |
NS NS NS NS NS NS NS NS NS NS NS |
13,298 3,458 1,390 7,833 0,618 18,323 0,460 8,508 3,350 6,005 6,558 |
0,147 0,045 0,020 0,156 0,009 0,403 0,016 0,196 0,086 0,155 0,080 |
<0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 <0,0001 |
0,0081 0,0081 0,0081 0,0081 0,0081 0,0081 0,0081 0,0081 0,0081 0,0081 0,0081 |
| Total DMAs dma16:0 dma18:0 dma18:1n-7 dma18:1n-9 |
6,395 1,870 2,570 1,190 0,765 |
0,108 0,024 0,065 0,017 0,016 |
6,833 1,978 2,985 1,215 0,655 |
0,015 0,032 0,027 0,005 0,010 |
NS NS NS NS NS |
5,850 1,770 2,845 0815 0420 |
0,127 0,036 0,076 0,010 0,009 |
0,0499 0,0499 0,0499 0,0499 0,0499 |
<0,0001 <0,0001 <0,0001 <0,0001 <0,0001 |
Finally, although the dietary supplement tested contains antioxidants (vitamin E, vitamin C, zinc, resveratrol and carotenoids (lutein and zeaxanthin)) beside fish oil, we think that the protection is mainly due to the fatty acids. Indeed, it had been shown that vitamin C pre-treatment of ARPE-19 cells in vitro reduced transcriptional activation of AP-1, a nuclear transcription factor involved in oxidative stress response, but at a concentration of 100 and 200 μM while we evaluated 13 μM of vitamin C at 11 μM eq. DHA and 105 μM of vitamin C at 44 μm eq. DHA of supplement. Vitamin E acts by both quenching oxy-radicals and recycling the α-tocopheroxyl radical resulting from vitamin E scavenging an oxy-radical[49]. Resveratrol has to be used between 25 μM and 100 μm to have an antioxidant protective effect against H2O2-induced cell death[50,51] whereas in the supplement the concentration reaches only 0.45 μm for at 44 μm eq. DHA of supplement. However, even at those low and separately ineffective concentrations, we can’t exclude synergic effect of these compounds which may confer additional protective effects by attenuating the propagation of oxidative stress[52-55].
| ID | Gene symbol | Description | Fold change | P-val | FDR P-val |
|---|---|---|---|---|---|
| TC0500012459.hg.1 | PDGFRB | Platelet-derived growth factor receptor, beta polypeptide | -1,87 | 2,14E-06 | 3,00E-04 |
| TC1700007791.hg.1 | RARA | Retinoid acid receptor, alpha | -1,78 | 2,13E-05 | 1,20E-03 |
| TC1200007819.hg.1 | CDK2 | Cyclin-dependent kinase 2 | -1,75 | 2,64E-06 | 3,00E-04 |
| TC2200007906.hg.1 | BID | BH3 interacting domain death agonist | -1,72 | 2,87E-05 | 1,50E-03 |
| TC1800008650.hg.1 | SMAD7 | SMAD family member 7 | -1,71 | 2,02E-05 | 1,20E-03 |
| TC2000006642.hg.1 | BMP2 | Bone morphogenetic protein 2 | -1,71 | 1,00E-04 | 4,10E-03 |
| TC0100011621.hg.1 | TGFB2; TGFB2-OT1 | Transforming growth factor beta 2; TGFB2 overlapping transcript 1 | -1,7 | 9,85E-07 | 2,00E-04 |
| TC0100010284.hg.1 | PEA15 | Phosphoprotein enriched in astrocytes 15 | -1,46 | 6,18E-05 | 2,40E-03 |
| TC1500009082.hg.1 | BMF | Bcl2 modifying factor | -1,45 | 4,00E-04 | 7,60E-03 |
| TC1200009876.hg.1 | CD69 | CD69 molecule | -1,43 | 3,65E-02 | 1,45E-01 |
| TC0100008664.hg.1 | GADD45A | Growth arrest and DNA-damage-inducible, alpha | -1,39 | 2,00E-04 | 5,00E-03 |
| TC1100006815.hg.1 | WEE1 | WEE1 G2 checkpoint kinase | -1,39 | 4,30E-03 | 3,74E-02 |
| TC2200007150.hg.1 | TIMP3 | TIMP metallopeptidase inhibitor 3 | -1,37 | 8,00E-04 | 1,25E-02 |
| TC1200006888.hg.1 | CDKN1B | Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | -1,37 | 2,70E-03 | 2,76E-02 |
| TC0200016682.hg.1 | PPP3R1 | Protein phosphatase 3, regulatory subunit B, alpha | -1,37 | 2,00E-03 | 2,26E-02 |
| TC1700011919.hg.1 | CEP295NL; TIMP2 | CEP295 N-terminal like; TIMP metallopeptidase inhibitor 2 | -1,36 | 7,00E-04 | 1,11E-02 |
| TC0800012001.hg.1 | PTK2 | Protein tyrosine kinase 2 | -1,35 | 1,40E-03 | 1,81E-02 |
| TC1600006652.hg.1 | TNFRSF12A | Tumor necrosis factor receptor superfamily, member 12A | -1,33 | 4,30E-03 | 3,76E-02 |
| TC1600009202.hg.1 | CREBBP | CREB binding protein | -1,33 | 4,50E-03 | 3,86E-02 |
| TC1200006909.hg.1 | EMP1 | Epithelial membrane protein 1 | -1,33 | 8,30E-03 | 5,72E-02 |
| TC0500007831.hg.1 | F2R | Coagulation factor II (thrombin) receptor | -1,3 | 1,50E-03 | 1,93E-02 |
| TC0500009706.hg.1 | SQSTM1 | Sequestosome 1 | 1,31 | 7,00E-04 | 1,15E-02 |
| TC1100012708.hg.1 | FEZ1 | Fasciculation and elongation protein zeta 1 | 1,31 | 4,10E-03 | 3,60E-02 |
| TC1000008388.hg.1 | FAS | Fas cell surface death receptor | 1,31 | 2,24E-02 | 1,07E-01 |
| TC0300013949.hg.1 | SATB1 | SATB homeobox 1 | 1,34 | 2,00E-04 | 5,10E-03 |
| TC1100007273.hg.1 | CD44 | CD44 molecule (Indian blood group) | 1,37 | 1,00E-04 | 3,80E-03 |
| TC1600011398.hg.1 | MMP2 | Matrix metallopeptidase 2 | 1,37 | 3,30E-03 | 3,13E-02 |
| TC0200013261.hg.1 | RETSAT | Retinol saturase (all-trans-retinol 13,14-reductase) | 1,37 | 6,10E-03 | 4,71E-02 |
| TC0900007576.hg.1 | ANXA1 | Annexin A1 | 1,38 | 1,23E-05 | 9,00E-04 |
| TC1200012643.hg.1 | ERBB3 | Erb-b2 receptor tyrosine kinase 3 | 1,38 | 2,46E-02 | 1,13E-01 |
| TC1200010968.hg.1 | DDIT3 | DNA-damage-inducible transcript 3 | 1,39 | 1,70E-03 | 2,05E-02 |
| TC0100013818.hg.1 | PPT1 | Palmitoyl-protein thioesterase 1 | 1,39 | 1,70E-03 | 2,04E-02 |
| TC1400007227.hg.1 | LGALS3 | Lectin, galactoside-binding, soluble, 3 | 1,44 | 4,00E-04 | 7,60E-03 |
| TC1800007471.hg.1 | PMAIP1 | Phorbol-12-myristate-13-acetate-induced protein 1 | 1,45 | 7,00E-03 | 5,11E-02 |
| TC0600013757.hg.1 | SOD2 | Superoxide dismutase 2, mitochondrial | 1,46 | 8,91E-05 | 3,00E-03 |
| TC0100008697.hg.1 | CTH | Cystathionine gamma-lyase | 1,51 | 9,46E-05 | 3,20E-03 |
| TC0700008873.hg.1 | CAV1 | Caveolin 1 | 1,51 | 3,70E-06 | 4,00E-04 |
| TCOX00006799.hg.1 | SAT1 | Spermidine/spermine N1-acetyltransferase 1 | 1,55 | 1,00E-03 | 1,42E-02 |
| TC0700011642.hg.1 | HGF | Hepatocyte growth factor (hepapoietin A; scatter factor) | 1,55 | 1,50E-03 | 1,86E-02 |
| TC1200007693.hg.1 | IGFBP6 | Insulin like growth factor binding protein 6 | 2,26 | 6,32E-08 | 3,65E-05 |
| TC0700006890.hg.1 | IL6 | Interleukin 6 | 2,81 | 3,44E-09 | 6,22E-06 |
| TC2200007204.hg.1 | HMOX1 | Heme oxygenase 1 | 3,8 | 2,29E-09 | 4,50E-06 |
Conclusion
We have shown that a complex ocular dietary supplement containing fish oil, antioxidants and resveratrol is associated in changes in fatty acid composition in ARPE- 19 cells. Although it protected the cells from H2O2-induced oxidative stress at 11 and 44 μM eq.DHA, the toxic effect at high concentration raised the question of the use of such a supplement over a long period of time in humans. Studies on fatty acid, antioxidants or any others compounds used in supplementation should be conducted over a long period to evaluate chronic toxicity in complement to acute toxicity.
Acknowledgment
None
Funding
The research was supported by CHU of Clermont-Ferrand, University of Clermont-Ferrand, CHR of Orléans, CNRS of Orléans, University of Orléans. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure Statement
None
Data Availability Statement
Data available on request from the authors.
Author's Contributions
Conceptualization: IRC; Data curation: KRBO, AM, AA, FL, SG, NA, OP, IRC; Formal analysis: KRBO, AM; Funding acquisition: IRC; Investigation: KRBO, AM; Methodology: AM, IRC; Project administration: IRC; Resources: IRC; Software: None; Supervision: IRC, OP; Validation: IRC, OP; Visualization: None; Roles/Writing-original draft: OP, IRC, AM; Writing-review and editing: KRBO, AM, AA, FL, SG, NA, OP, IRC.
References
- Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, Mccarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572.
[Crossref] [Google Scholar] [Indexed]
- Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: The beaver dam eye study. Ophthalmology. 1992;99(6):933-943.
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738.
- Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(13):2724-2735.
- Green WR, Mcdonnell PJ, Yeo JH. Pathologic features of senile macular degeneratlon. Ophthalmology. 1985;92(5):615-627.
- Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989;30(8):1691-1699.
- Bhutto I, Lutty G. Understanding Age-Related Macular Degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med. 2012;33(4):295-317.
- Walchuk C, Suh M. Nutrition and the aging retina: A comprehensive review of the relationship between nutrients and their role in age-related macular degeneration and retina disease prevention. Adv Food Nutr Res. 2020;93:293-332.
[Crossref] [Google Scholar] [Indexed]
- Ardourel M, Felgerolle C, Pâris A, Acar N, Ramchani Ben Othman K, Ueda N, et al. Dietary supplement enriched in antioxidants and omega-3 promotes glutamine synthesis in Müller cells: A key process against oxidative stress in retina. Nutrients. 2021;13(9):3216.
- Ramchani-Ben Othman K, Cercy C, Amri M, Doly M, Ranchon-Cole I. Dietary supplement enriched in antioxidants and omega-3 protects from progressive light-induced retinal degeneration. Plos One. 2015;10(6): e0128395.
[Crossref] [Google Scholar] [Indexed]
- Lewandowski D, Sander CL, Tworak A, Gao F, Xu Q, Skowronska-Krawczyk D. Dynamic lipid turnover in photoreceptors and retinal pigment epithelium throughout life. Prog Retin Eye Res. 2022;89:101037.
[Crossref] [Google Scholar] [Indexed]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845-881
[Crossref] [Google Scholar] [Indexed]
- Ao J, Wood JP, Chidlow G, Gillies MC, Casson RJ. Retinal pigment epithelium in the pathogenesis of age‐related macular degeneration and photobiomodulation as a potential therapy?. Clin Exp Ophthalmol. 2018;46(6):670-686.
- Kaarniranta K, Salminen A, Eskelinen EL, Kopitz J. Heat shock proteins as gatekeepers of proteolytic pathways-implications for Age-Related Macular Degeneration (AMD). Ageing Res Rev. 2009;8(2):128-139.
[Crossref] [Google Scholar] [Indexed]
- Plafker SM, O'Mealey GB, Szweda LI. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int Rev Cell Mol Biol. 2012;298:135-177.
[Crossref] [Google Scholar] [Indexed]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62(2):155-170.
[Crossref] [Google Scholar] [Indexed]
- Kaczara P, Sarna T, Burke JM. Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress. Free Radic Biol Med. 2010;48(8):1064-1070.
[Crossref] [Google Scholar] [Indexed]
- Weigel AL, Handa JT, Hjelmeland LM. Microarray analysis of H2O2-, HNE-, or tBH-treated ARPE-19 cells. Free Radic Biol Med. 2002;33(10):1419-1432. [Crossref]
- Miceli MV, Liles MR, Newsome DA. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp Cell Res. 1994;214(1):242-249.
- Tate D, Miceli MV, Newsome DA. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995;36(7):1271-1279.
- Kostadinova R, Boess F, Applegate D, Suter L, Weiser T, Singer T, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol. 2013;268(1):1-6.
[Crossref] [Google Scholar] [Indexed]
- Rodrigues PM, Afonso MB, Simão AL, Borralho PM, Rodrigues CM, Castro RE. Inhibition of NF-κB by deoxycholic acid induces miR-21/PDCD4-dependent hepatocellular apoptosis. Sci Rep. 2015;5(1):17528.
[Crossref] [Google Scholar] [Indexed]
- Rodriguez GE, Torriglia A. Calpain 1 induce lysosomal permeabilization by cleavage of lysosomal associated membrane protein 2. Biochim Biophys Acta. 2013;1833(10):2244-2253.
[Crossref] [Google Scholar] [Indexed]
- Ardourel M, Pâris A, Felgerolle C, Lesne F, Ranchon-Cole I, Briault S, et al. FMRP-related retinal phenotypes: Evidence of glutamate-glutamine metabolic cycle impairment. Exp Eye Res. 2022; 224:109238.
[Crossref] [Google Scholar] [Indexed]
- Kagan VE, Fabisiak JP, Shvedova AA, Tyurina YY, Tyurin VA, Schor NF, et al. Oxidative signaling pathway for externalization of plasma membrane phosphatidylserine during apoptosis. FEBS Lett. 2000;477(1-2):1-7.
[Crossref] [Google Scholar] [Indexed]
- Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: Implications for apoptosis. J Biol Chem. 2007;282(25):18357-18364. [Crossref]
- Bikulčienė I, Golubevaitė O, Žėkas V, Radzevičius M, Karčiauskaitė D, Matuzevičienė R, et al. Association of platelet membrane fatty acid composition with markers of oxidative stress in healthy men. Med Sci Monit. 2019;25:6405.
[Crossref] [Google Scholar] [Indexed]
- Brown ER, Subbaiah PV. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on human skin fibroblasts. Lipids. 1994;29(12):825-829.
[Crossref] [Google Scholar] [Indexed]
- Calder PC, Yaqoob P, Harvey DJ, Watts A, Newsholme EA. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem J. 1994;300(2):509-518.
[Crossref] [Google Scholar] [Indexed]
- Sobajima T, Tamiya-Koizumi K, Ishihara H, Kojima K. Effects of fatty acid modification of ascites tumor cells on pulmonary metastasis in rat. Jpn J Cancer Res. 1986;77(7):657-63.
[Crossref] [Google Scholar] [Indexed]
- Yorek M, Leeney E, Dunlap J, Ginsberg B. Effect of fatty acid composition on insulin and IGF-I binding in retinoblastoma cells. Invest Ophthalmol Vis Sci. 1989;30(10):2087-2092.
- Armstrong VT, Brzustowicz MR, Wassall SR, Jenski LJ, Stillwell W. Rapid flip-flop in polyunsaturated (docosahexaenoate) phospholipid membranes. Arch Biochem Biophys. 2003;414(1):74-82.
- Roberts LJ, Milne GL. Isoprostanes. J Lipid Res. 2009;50:S219-S223.
[Crossref] [Google Scholar] [Indexed]
- Milne GL, Dai Q, Roberts II LJ. The isoprostanes-25 years later. Biochim Biophys Acta. 2015;1851(4):433-445.
[Crossref] [Google Scholar] [Indexed]
- Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts 2nd LJ. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci. 1992;89(22):10721-10725.
[Crossref] [Google Scholar] [Indexed]
- Morrow JD, Awad JA, Kato T, Takahashi K, Badr KF, Roberts LJ, et al. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992;90(6):2502-2507.
[Crossref] [Google Scholar] [Indexed]
- Brooks JD, Milne GL, Yin H, Sanchez SC, Porter NA, Morrow JD. Formation of highly reactive cyclopentenone isoprostane compounds (A3/J3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2008 ;283(18):12043-12055.
[Crossref] [Google Scholar] [Indexed]
- Yin Y, Zhan WH, Peng JS, Zhao ZG. Apoptosis of human gastric cancer cells induced by omega-3 polyunsaturated fatty acids. Zhonghua Wei Chang. 2007;10(6):570-573.
- Mccappin SC, Vandongen R, Croft KD. The effect of dietary eicosapentaenoic acid on arachidonic acid incorporation and metabolism in rat leukocytes. Prostaglandins. 1987;34(4):505-517.
- Adam AC, Lie KK, Moren M, Skjærven KH. High dietary arachidonic acid levels induce changes in complex lipids and immune-related eicosanoids and increase levels of oxidised metabolites in zebrafish (Danio rerio). Br J Nutr. 2017;117(8):1075-1085.
[Crossref] [Google Scholar] [Indexed]
- Dong Y, Wei Y, Wang L, Song K, Zhang C, Lu K, et al. Dietary n-3/n-6 polyunsaturated fatty acid ratio modulates growth performance in spotted seabass (Lateolabrax maculatus) through regulating lipid metabolism, hepatic antioxidant capacity and intestinal health. Anim Nutr. 2023; 14:20-31.
[Crossref] [Google Scholar] [Indexed]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278(44):43807-438017.
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci. 2004;101(22):8491-8496.
[Crossref] [Google Scholar] [Indexed]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24(1):87-138.
[Crossref] [Google Scholar] [Indexed]
- Paul S, Lancaster GI, Meikle PJ. Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog Lipid Res. 2019;74:186-195.
[Crossref] [Google Scholar] [Indexed]
- Chow SC, Sisfontes L, Björkhem I, Jondal M. Suppression of growth in a leukemic T cell line by n-3 and n-6 polyunsaturated fatty acids. Lipids. 1989;24:700-704.
[Crossref] [Google Scholar] [Indexed]
- Morandat S, Bortolato M, Anker G, Doutheau A, Lagarde M, Chauvet JP, et al. Plasmalogens protect unsaturated lipids against UV-induced oxidation in monolayer. Biochim Biophys Acta. 2003;1616(2):137-146.
[Crossref] [Google Scholar] [Indexed]
- Knott EJ, Gordon WC, Jun B, Do K, Bazan NG. Retinal pigment epithelium and photoreceptor preconditioning protection requires docosanoid signaling. Cell Mol Neurobiol. 2018; 38:901-917.
- Lien EL, Hammond BR. Nutritional influences on visual development and function. Prog Retin Eye Res. 2011;30(3):188-203.
[Crossref] [Google Scholar] [Indexed]
- King RE, Kent KD, Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem Biol Interact. 2005;151(2):143-149.
[Crossref] [Google Scholar] [Indexed]
- Pintea A, Rugină D, Pop R, Bunea A, Socaciu C, Diehl HA. Antioxidant effect of trans-resveratrol in cultured human retinal pigment epithelial cells. J Ocul Pharmacol Ther. 2011;27(4):315-321. [Crossref]
- Roig-Pérez S, Guardiola F, Moretó M, Ferrer R. Lipid peroxidation induced by DHA enrichment modifies paracellular permeability in Caco-2 cells: Protective role of taurine. J Lipid Res. 2004;45(8):1418-1428.
[Crossref] [Google Scholar] [Indexed]
- Jain SK. In vivo externalization of phosphatidylserine and phosphatidylethanolamine in the membrane bilayer and hypercoagulability by the lipid peroxidation of erythrocytes in rats. J Clin Invest. 1985;76(1):281-286.
- Dugas B, Charbonnier S, Baarine M, Ragot K, Delmas D, Ménétrier F, et al. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: Cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur J Nutr. 2010;49:435-446.
[Crossref] [Google Scholar] [Indexed]
- Wrona M, Różanowska M, Sarna T. Zeaxanthin in combination with ascorbic acid or α-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic Biol Med. 2004;36(9):1094-1101.
[Crossref] [Google Scholar] [Indexed]
Author Info
Maryvonne ARDOUREL1, Khaoula RAMCHANI-BEN OTHMAN2, Amir ATTALLAH3, Fabien LESNE1, Stéphane GRÉGOIRE1, Niyazi ACAR4, Olivier PERCHE1* and Isabelle Ranchon COLE21Department of Neurosciences Research, Clermont-Ferrand University, Clermont-Ferrand, UMR 1107, NeuroDol, France
2Department of Molecular and Experimental Immunology and Neurogenetics, Orléans University, Orléans, France
3Department of Innovation and Health Research, Orleans University, Orleans, France
4Department of Nutrition, University of Bourgogne, Dijon, France
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Google Scholar citation report
Citations : 2439
Clinical Nutrition and Hospital Dietetics received 2439 citations as per google scholar report
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- SCOPUS
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Web of Science
Journal Highlights
- Blood Glucose
- Dietary Supplements
- Cholesterol, Dehydration
- Digestion
- Electrolytes
- Clinical Nutrition Studies
- energy balance
- Diet quality
- Clinical Nutrition and Hospital Dietetics