Personalized dietary therapy for prevention of dementia using AI
Review Article - (2024) Volume 0, Issue 0
Received: 26-Feb-2024, Manuscript No. CNHD-24-128298; Editor assigned: 28-Feb-2024, Pre QC No. CNHD-24-128298 (PQ); Reviewed: 13-Mar-2024, QC No. CNHD-24-128298; Revised: 20-Mar-2024, Manuscript No. CNHD-24-128298 (R); Published: 27-Mar-2024, DOI: 10.12873/0211-6057.44.S1.001
Abstract
Recent studies suggest that systemic metabolic disorders may lead to cognitive impairment and potentially increase the risk of dementia onset. Specifically, lifestyle diseases an contribute to vascular cognitive impairment resulting from atherosclerosis. Moreover, other systemic metabolic factors, such as malnutrition, can also influence the risk of cognitive impairment. Based on the relationship between the brain and systemic metabolism, we utilized AI technology to assess the risk of dementia using health check-up data including basic blood data that did not include dementia-specific biomarkers. The AI model was trained to estimate cognitive function, represented by the Mini-Mental State Examination (MMSE) from basic blood test data and age. The trained AI model was able to estimate MMSE scores with high accuracy using blood test data and subject’s ages as inputs. Although the estimation accuracy slightly decreased when age was excluded from the input features, a significant correlation with the actual measurements was still observed. The most important variables in the estimation were subject’s ages, followed by blood parameters indicating malnutrition, anemia, renal function, and liver function. These variables showed significant correlations with MMSE. Such findings suggest that the pathology of dementia should not be limited to the brain alone, but rather should be considered as a systemic metabolic disorder. By considering dementia in this way, it becomes possible to evaluate the risk of dementia using health check-ups. Furthermore, it becomes possible to identify metabolic disorders underlying an individual’s dementia risk based on abnormalities in blood data and provide personalized dietary interventions accordingly. Currently, dietary therapies aimed at preventing cognitive impairment are mainly uniform diets intended for the prevention of lifestyle diseases, such as the Mediterranean diet. However, combining individualized dietary therapies tailored to metabolic disorders contributing to an individual’s dementia risk may offer a more effective approach to dementia prevention.
Keywords
Alzheimer’s disease, Artificial intelligence, Deep learning, Dementia, Dietary therapy, Health check-up, Nutrition
Introduction
With the aging of society, the number of patients with dementia, such as Alzheimer’s Disease (AD), is rapidly increasing, becoming a serious social issue [1]. However, drugs that can treat or prevent dementia, such as Alzheimer’s disease, at the molecular level are still under development. Meanwhile, improvements in lifestyle habits centered around dietary therapy are gaining attention as an important approach to dementia prevention. For example, the Mediterranean diet is known to have effects on inhibiting arteriosclerosis and reports also suggest that it has protective effects on cognitive function [2]. Furthermore, the MIND diet, which adds the DASH diet (Dietary Approaches to Stop Hypertension) to the Mediterranean diet, has been developed for the purpose of preventing dementia [3].
Arteriosclerosis, caused by lifestyle diseases such as diabetes mellitus, hypertension, and lipid metabolic disorders, plays an important role not only in cardiovascular disorders but also in Vascular Cognitive Impairment (VCI). VCI significantly contributes to cognitive disorders, ranging from Mild Cognitive Impairment (MCI) to severe dementia [4-6]. Notably, VCI contributes not only to vascular dementia but also to Alzheimer’s Disease (AD) in the elderly [7]. Furthermore, non-lifestyle-related metabolic disorders such as malnutrition [8], anemia [9], liver diseases [10], and kidney diseases [11] can cause cognitive impairment and increase the risk of dementia. It is important to note that dietary therapy for arteriosclerosis is not effective against these non-lifestyle-related metabolic disorders, and these disorders vary from individual to individual.
These findings suggest the need for personalized dietary therapies aimed at preventing the onset of dementia, tailored to accommodate each individual’s specific metabolic disorders that contribute to cognitive risk. This paper introduces a screening test for assessing dementia risk through the analysis of health checkup data using Artificial Intelligence (AI), recently developed by Sakatani et al. [12-16]. Following this, a detailed explanation of the individualized dietary therapy for dementia prevention, utilizing this AI-based screening test, will be provided. Combining such AI-based individualized dietary therapy with a comprehensive diet like the MIND diet is anticipated to improve the effectiveness of dementia prevention.
Literature Review
AI-based risk assessment of cognitive impairment
Basic principle for assessing cognitive impairment risk using AI: The dementia risk assessment method using AI analysis is based on the role of systemic metabolic disorders, both lifestyle-related and non-lifestyle-related, in the development of cognitive disorders [16]. Health checkup data, such as blood data, including Complete Blood Count (CBC) and Basic Metabolic Panel (BMP), reflect these systemic metabolic disorders. By analyzing these health checkup data through AI analysis (i.e., deep learning), the risk of developing cognitive disorders can be quantitatively estimated [16]. This allows for the implementation of individualized nutritional guidance based on the analysis of personal risk factors (Figure 1).
Figure 1. Conceptual Diagram of AI-based risk assessment of cognitive impairment. Lifestyle diseases like diabetes can lead to arteriosclerosis and Vascular Cognitive Impairment (VCI), increasing the risk of dementia. Non-lifestyle-related metabolic disorders, such as malnutrition, anemia, renal, and liver dysfunction, also contribute to this risk. These metabolic disorders can be detected via health check-up data, including basic blood test data. Using AI, we predict dementia risk from health check-up data, identifying individual risk factors, and offering personalized dietary therapy
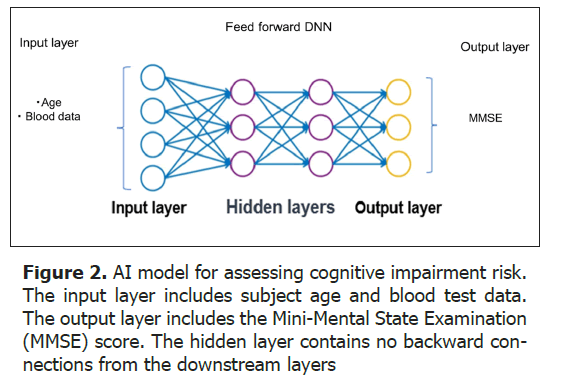
For the AI analysis of health examination data, we developed a feedforward Deep Neural Network (DNN) model (Figure 2) [16]. The input layer included 23 blood test items from basic blood tests as well as age, while the output layer inputted the Mini Mental State Examination (MMSE) score. By using MRI brain atrophy indices as the output layer, the degree of brain atrophy can also be estimated from blood data [13]. The blood test items are shown in Table 1. Note that dementia-specific biomarkers, such as amyloid-beta, are not included. To date, the DNN model has been trained on the relationship between input data (blood data, age) and cognitive function MMSE using a total of 2,538 subjects. For the validation and application of the DNN model, 37,861 cases have been utilized.
| Complete blood count | General biochemical examination | |
|---|---|---|
| WBC count | Total protein | BUN |
| RBC count | Albumin | Creatinine |
| Hemoglobin | A/G ratio | Uric acid |
| Hematocrit | AST (GOT) | Glucose |
| MCV | ALT (GPT) | Na |
| MCH | r-GTP | K |
| MCHC | Total cholesterol | CI |
| Platelet count | Triglyceride | |
| Note: MCV: Mean Corpuscular Volume; MCH: Mean Corpuscular Hemoglobin; MCHC: Mean Corpuscular Hemoglobin Concentration; BUN: Blood Urea Nitrogen | ||
Verification of estimation accuracy of the DNN model for cognitive impairment risk: The estimation accuracy of the DNN model for cognitive impairment risk was evaluated (Figure 3) [16]. The estimated MMSE scores obtained through Leave-One-Out Cross-Validation (LOOCV) within the training group showed a high correlation with the actual MMSE scores (r=0.85, p<0.001). When estimated using only blood data without age in the input layer to exclude the influence of age, the correlation coefficient slightly decreased but still showed a high correlation (r=0.75, p<0.001). Next, when testing estimation accuracy in a test group comprised of subjects not used in the learning phase, the correlation coefficient was 0.66 (p<0.001), indicating a moderate correlation.
Figure 3. Correlation between estimated and actual MMSE scores. A: The estimated MMSE scores through LOOCV showed a high correlation with the actual MMSE scores (r=0.85, p<0.001) B: When estimating without including age in the input layer, the correlation coefficient still exhibited a high correlation (r=0.75, p<0.001) (B). C: When validating the estimation accuracy on a test group, the correlation coefficient was r=0.66 (p<0.001), indicating a moderate correlation. The horizontal axis represents the measured MMSE score, while the vertical axis indicates the MMSE score predicted by the AI-based screening test
Furthermore, when validating the test group with a twoclass classification of MMSE (normal above 24 points, suspected dementia below 23 points), a high evaluation accuracy with a specificity of 87% and a sensitivity of 75% was observed [16].
Evaluation of exercise and dietary therapy effects using the DNN model: We investigated whether the DNN model could be applied to monitor the effectiveness of health promotion activities such as dietary and/or exercise therapies on cognitive function [15]. The effects of a three-month exercise therapy on members of a fitness gym for the elderly (n=7, 68.6 ± 3.2 years) were evaluated using the DNN model. A significant improvement in MMSE scores estimated by the DNN model was observed after the three months of exercise therapy (p=0.024).
Additionally, we studied a total of 230 subjects (67.9 ± 7.4 years old) who were members of a health promotion class [15]. The subjects were divided into four groups based on their MMSE scores evaluated by the DNN model: Class A (28 ≤ x ≤ 30, n=183), B (24 ≤ x <28, n=44), C (20 ≤ x <24, n=2), and D (x <20, n=1). After the two-month intervention, 46 cases showed an increase in functional classes, with mean MMSE scores rising from 25.6 ± 2.0 to 26.7 ± 1.4 (p=0.0003). Conversely, 20 cases showed a decrease in functional classes, with mean MMSE scores dropping from 27.7 ± 0.7 to 26.3 ± 0.5 (p=0.000). In Class A (n=163), mean MMSE scores increased from 28.4 ± 1.1 to 28.6 ± 1.2 (p=0.017). The MMSE scores predicted by the DL-based test increased in most subjects following the exercise-diet therapy. The DNN model may be useful for monitoring the effects of exercise- diet therapy on the risk of cognitive impairment in elderly people.
Personalized dietary therapy
Dietary therapy for non-lifestyle-related metabolic disorders: Currently, the prevention of dementia focuses primarily on preventing arteriosclerosis caused by lifestyle diseases. This is because arteriosclerosis significantly contributes to the development of cognitive impairments as Vascular Cognitive Impairment (VCI) [4-7].
However, it has become clear that non-lifestyle diseases also lead to cognitive impairments, becoming a risk factor for dementia [8-11]. The primary non-lifestyle-related metabolic disorders reported to pose a risk for the onset of dementia include malnutrition [8], anemia [9], liver diseases [10], and kidney diseases [11].
It is important to note that the non-lifestyle-related meta-bolic disorders require dietary therapies which differ from those for lifestyle diseases. For example, dietary therapy for conditions such as anemia and hypoalbuminemia, which are forms of malnutrition commonly seen in frail elderly individuals, differs from that for lifestyle diseases. Namely, dietary therapy for lifestyle diseases focuses on reducing intake of carbohydrates and fats, whereas in contrast, dietary plans for frailty in the elderly emphasize protein-rich and nutrient-dense foods to combat malnutrition and muscle loss, prioritizing overall nutritional improvement and maintenance of physical capacity [17]. On one hand, the Mediterranean diet, known for preventing lifestyle diseases, has also been reported to be effective in preventing frailty [18-20]. These observations suggest that combining the Mediterranean diet with personalized dietary therapy is to be effective in preventing the onset of dementia.
In recent years, it has been recognized that renal and liver dysfunction can affect the brain through the kidney-brain axis [21,22] and liver-brain axis [23], respectively, leading to cognitive impairments. Dietary therapy for renal and liver dysfunction also differs from those of lifestyle diseases. Dietary therapy for renal involves restricting protein to lessen kidney strain, managing potassium and phosphorus to avoid imbalances, reducing sodium to control hypertension and swelling, and regulating water intake based on the disease stage. A balanced diet including vegetables, fruits, and whole grains is recommended. Personalized dietary management with healthcare professional guidance is crucial. Liver dysfunction often necessitates limiting protein and salt intake due to concerns like uremia and ammonia build up, whereas lifestyle disease diets focus more on managing carbohydrate intake, particularly refined carbs, and overall calorie control. In addition, alcohol consumption is strictly limited or avoided in liver conditions but may be moderately acceptable in managing lifestyle diseases. Liver-focused diets address unique issues like toxin accumulation, while lifestyle disease diets prioritize blood sugar control and cardiovascular risk reduction. Considering the complexity of non-lifestyle-related metabolic disorders, adopting a personalized dietary therapy is essential. Such treatment should be based on a thorough understanding of each individual’s health condition and metabolic abnormalities. This individualized approach not only addresses each person’s unique nutritional needs but also has the potential to enhance the effectiveness of preventing dementia onset.
AI-based personalized dietary therapy: The input layer of the DNN model utilizes basic blood data (see Table 2) that reflect systemic metabolic disorders. This allows for the identification of patterns in metabolic disorders, which are risk factors for cognitive impairment in individual subjects. Namely, it identifies conditions such as anemia (red blood cell count, Hb, HT), nutritional disorders (total protein, albumin, A/G ratio), liver dysfunction (AST, ALT, γGTP), renal dysfunction (BUN, creatinine), lipid metabolism abnormalities (total cholesterol, LDL-CH, HDL-CL, triglycerides), diabetes (blood glucose level), gout (uric acid), and electrolyte abnormalities (Na, Cl, K). This enables the advantage of providing personalized, tailor-made nutritional guidance for each individual. Table 2 shows the relationship between metabolic disorders that pose a risk for the onset of dementia and blood test parameters. Recently, we developed a method to mathematically estimate the contribution of each test item to the risk of cognitive impairment using a DNN model. Blood data in a CSV file (Figure 4, upper section) is analyzed using a proprietary algorithm, with the contribution shown as red (positive direction) and blue (negative direction) markers (Figure 4 and Table 2).
| Metabolic disorders associated with dementia risk | Blood data |
|---|---|
| Anemia | RBC, Hemoglobin, Hematocrit |
| Malnutrition | T Protein, Albumin, A/G ratio |
| Lipid metabolic disorder | T Cholesterol, Triglyceride (HDL-C, LDL-C) |
| Renal dysfunction | Creatinine, BUN |
| Liver dysfunction | AST, ALT, r-GTP |
| Diabetes mellitus | Glucose |
| Serum electrolyte imbalance | Na, Cl, K |
| Note: (HDL-C, LDL-C): Currently included in the input features of the DNN model | |
Figure 4. Contribution of blood test items to the risk of cognitive impairment. The upper section contains blood data from four cases in a CSV file. The lower section shows the contribution of each blood data item to the estimated MMSE score (red indicates a positive influence on the estimated value, and blue indicates a negative influence)
Figure 5 illustrates the flow of cognitive impairment risk assessment and dietary guidance using the AI-based screening test at a health check-up center. After receiving a general health check-up including blood sampling, the recipient undergoes the AI-based screening test using their health check-up data. If the assessment result indicates a risk of cognitive impairments (estimated MMSE score less than 27), the next step involves various dementia tests including MMSE and MRI. For individuals desiring further evaluation for Alzheimer’s disease, amyloid PET scans will be conducted. If dementia is diagnosed, a consultation with a specialist will follow. Meanwhile, metabolic disorders contributing to the risk of dementia will be identified based on the contribution of test items. A dietary therapy tailored to those metabolic disorders will be suggested. These personalized dietary therapies are primarily provided to cases diagnosed with MCI and are implemented in conjunction with exercise therapy (Figure 5).
Discussion
The present AI-based method quantitatively estimates cognitive impairment risk from systemic metabolic disorders using MMSE scores. It identifies systemic metabolic abnormalities prone to cognitive impairment, targeting prevention through dietary and lifestyle changes. It should be noted that the present method isn’t a direct cognitive function assessment but indicates cognitive impairment risk using MMSE scores. The present AI-based method can be used not only in hospitals and care facilities but also in non-medical settings like sports gyms and health classes. It allows for personal cognitive impairment risk assessment and individual dietary therapy via smartphones. The AI-based method uses only health check-up data, making it applicable to dementia insurance in life insurance policies. Dementia insurance, part of long-term care insurance plans covering dementia-related care and services, can benefit from the risk assessment and preventive dietary therapy, potentially preventing dementia onset in policyholders. Collaborating with insurance companies could enable large-scale dementia prevention using the AI-based method. The following points summarize the advantages of the present method:
• It doesn’t require new blood samples and provides quick analysis results.
• There is no need for equipment to measure specific biomarkers for dementia, and the testing costs are low.
• Suitable for mass screening of cognitive impairment.
• Past health examination results can be used to determine cognitive impairment risk at that time.
• Cognitive impairment risk can be assessed conveniently using smartphones.
Finally, it’s necessary to discuss the challenges of the current research. Firstly, the model’s input layer primarily uses only blood data and participant age, not including physical findings (such as BMI, blood pressure) or medical history. Expanding the input items could improve prediction accuracy. Secondly, there’s a need to increase the number of cases used for training, and to include cases with various cognitive function levels and types of metabolic disorders. Thirdly, the current study uses Japanese data for DNN model training, but development using data from countries with different lifestyles is needed. Lastly, research directions include studies for large-scale prevention of cognitive impairment and studies clarifying the relationship between cognitive impairment and systemic metabolic disorders. The former requires research to enhance accuracy with fewer blood tests to reduce testing costs, while the latter may need studies combining various biomarkers and imaging tests. Further research is needed to overcome these limitations to establish the current method as a preventative approach for dementia onset.
Conclusion
We developed an AI-based dementia risk screening test using health check-up data aimed at preventing the onset of cognitive impairment in the elderly. This method is based on the concept that lifestyle diseases, as well as non-lifestyle-related metabolic disorders such as nutritional disorders, are risk factors for cognitive impairment. It uses AI to analyze health check-up data including basic blood test data that reflect these metabolic disorders, quantitatively estimates the risk of cognitive impairment, and provides individualized nutritional guidance based on each person’s risk factors. This approach allows for personalized dietary therapy tailored to individual metabolic disorders. By integrating individualized nutritional guidance with general dietary therapies like the MIND diet, we believe enhanced effectiveness in dementia prevention. This research highlights the potential of dietary therapy and AI utilization in dementia prevention.
References
- Stephan B, Birdi R, Tang EY, Cosco TD, Donini LM, Licher S, et al. Secular trends in dementia prevalence and incidence worldwide: A systematic review. J Alzheimers Dis. 2018; 66(2):653-680.
[Crossref] [Google Scholar] [Indexed]
- Román GC, Jackson RE, Gadhia R, Román AN, Reis J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; Polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; Probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol. 2019; 175(10):724-741.
[Crossref] [Google Scholar] [Indexed]
- Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015; 11(9):1015-1022.
[Crossref] [Google Scholar] [Indexed]
- van Der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CL, et al. Vascular cognitive impairment. Nat Rev Dis Primers. 2018; 4(1):1-6.
[Crossref] [Google Scholar] [Indexed]
- Al-Qazzaz NK, Ali SH, Ahmad SA, Islam S, Mohamad K. Cognitive impairment and memory dysfunction after a stroke diagnosis: A post-stroke memory assessment. Neuropsychiatr Dis Treat. 2014:1677-1691.
[Crossref] [Google Scholar] [Indexed]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42(9):2672-2713.
[Crossref] [Google Scholar] [Indexed]
- Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, et al. White matter hyperintensities and cerebral amyloidosis: Necessary and sufficient for clinical expression of Alzheimer disease?. JAMA Neurol. 2013; 70(4):455-461.
[Crossref] [Google Scholar] [Indexed]
- Sugimoto T, Arai H, Sakurai T. An update on cognitive frailty: Its definition, impact, associated factors and underlying mechanisms, and interventions. Geriatr Gerontol Int. 2022 ; 22(2):99-109.
- Hong CH, Falvey C, Harris TB, Simonsick EM, Satterfield S, Ferrucci L, et al. Anemia and risk of dementia in older adults: Findings from the Health ABC study. Neurology. 2013; 81(6):528-533.
[Crossref] [Google Scholar] [Indexed]
- Kunutsor SK, Laukkanen JA. Gamma glutamyltransferase and risk of future dementia in middle-aged to older finnish men: A new prospective cohort study. Alzheimers Dement. 2016; 12(9):931-941.
[Crossref] [Google Scholar] [Indexed]
- Tsuruya K, Yoshida H. Brain atrophy and cognitive impairment in chronic kidney disease. Contrib Nephrol. 2018; 196: 27-36. [Crossref]
- Karako K, Chen Y, Oyama K, Hu L, Sakatani K. Relationship between cognitive function, oral conditions and systemic metabolic function in the elderly. Adv Exp Med Biol.2022; 27-31.
[Crossref] [Google Scholar] [Indexed]
- Sakatani K, Oyama K, Hu L, Warisawa SI. Estimation of human cerebral atrophy based on systemic metabolic status using machine learning. Front Neurol. 2022; 13: 869915.
[Crossref] [Google Scholar] [Indexed]
- Oyama K, Sakatani K. Machine learning-based assessment of cognitive impairment using time-resolved near-infrared spectroscopy and basic blood test. Front Neurol. 2022; 12:624063.
[Crossref] [Google Scholar] [Indexed]
- Sakatani K, Oyama K, Hu L, Warisawa S, Yamashita T. Effects of exercise-diet therapy on cognitive function in healthy elderly people evaluated by deep learning based on basic blood test data. Adv Exp Med Biol. 2022:139-143.
[Crossref] [Google Scholar] [Indexed]
- Sakatani K, Oyama K, Hu L. Deep learning-based screening test for cognitive impairment using basic blood test data for health examination. Front Neurol. 2020; 11:588140.
[Crossref] [Google Scholar] [Indexed]
- Lochlainn MN, Cox NJ, Wilson T, Hayhoe RP, Ramsay SE, Granic A, et al. Nutrition and frailty. Nutrients. 2021; 13:7.
[Crossref] [Google Scholar] [Indexed]
- Dominguez LJ, Donat-Vargas C, Sayon-Orea C, Barberia-Latasa M, Veronese N, Rey-Garcia J, et al. Rationale of the association between Mediterranean diet and the risk of frailty in older adults and systematic review and meta-analysis. Exp Gerontol. 2023; 177:112180.
[Crossref] [Google Scholar] [Indexed]
- Poursalehi D, Lotfi K, Saneei P. Adherence to the Mediterranean diet and risk of frailty and pre-frailty in elderly adults: A systematic review and dose-response meta-analysis with GRADE assessment. Ageing Res Rev. 2023:101903.
[Crossref] [Google Scholar] [Indexed]
- Coelho-Júnior HJ, Trichopoulou A, Panza F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res Rev. 2021; 70:101395.
[Crossref] [Google Scholar] [Indexed]
- Xie Z, Tong S, Chu X, Feng T, Geng M. Chronic kidney disease and cognitive impairment: The kidney-brain axis. Kidney Dis (Basel). 2022; 8(4):275-85.
[Crossref] [Google Scholar] [Indexed]
- Stanciu GD, Ababei DC, Bild V, Bild W, Paduraru L, Gutu MM, et al. Renal contributions in the pathophysiology and neuropathological substrates shared by chronic kidney disease and Alzheimer’s disease. Brain Sci. 2020; 10(8):563.
[Crossref] [Google Scholar] [Indexed]
- Matsubara Y, Kiyohara H, Teratani T, Mikami Y, Kanai T. Organ and brain crosstalk: The liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacology. 2022; 205:108915.
[Crossref] [Google Scholar] [Indexed]
Author Info
Kaoru SAKATANI1,2*, Seika KAMOHARA2,3, Kenji KARAKO2 and Katsunori OYAMA42Department of Human and Engineered Environmental Studies, The University of Tokyo, Tokyo, Japan
3Department of Health and Nutrition, University of Human Arts and Sciences, Saitama, Japan
4Department of Computer Science, Nihon University College of Engineering, Tokyo, Japan
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Google Scholar citation report
Citations : 2439
Clinical Nutrition and Hospital Dietetics received 2439 citations as per google scholar report
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- SCOPUS
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Web of Science
Journal Highlights
- Blood Glucose
- Dietary Supplements
- Cholesterol, Dehydration
- Digestion
- Electrolytes
- Clinical Nutrition Studies
- energy balance
- Diet quality
- Clinical Nutrition and Hospital Dietetics