Protective effects of Sardinella maderensis oil on cardiometabolic risks in obese rat
Research Article - (2024) Volume 0, Issue 0
Received: 30-May-2024, Manuscript No. CNHD-24-137613; Editor assigned: 03-Jun-2024, Pre QC No. CNHD-24-137613 (PQ); Reviewed: 17-Jun-2024, QC No. CNHD-24-137613; Revised: 24-Jun-2024, Manuscript No. CNHD-24-137613 (R); Published: 01-Jul-2024, DOI: 10.12873/0211-6057.44.S1.005.
Abstract
Dietary habits with sea food containing high polyunsaturated fatty acid content have been associated with a low risk of developing obesity. Obesity is associated with increased cardiometabolic risks due to increased copying of western diet and sedentary lifestyle. This research aimed at evaluating the protective effect of Sardinella maderensis oil on obesity, insulin resistance and biomarkers of cardiometabolic dysfunction in obese albino rats receiving high fat diet. The fish oil was extracted by the Bligh and Dyer method followed by analysis of oil quality indices, Percentage Free Fatty Acids (%FFA), Iodine Value (IV), Peroxide Value (PV), p-Anisidine Value (p-AV) and Total Oxidation Value (TOTOX) using the standard methods of the AOAC (Association of Official Analytical Chemists). An ethical clearance was obtained in order to carry out in vivo studies. Thirty-six male albino rats were randomly and evenly distributed into six groups of six animals each. These groups were: Normal group (healthy rats fed with normal rodent chow), negative control (obese+250 mg/ kg body weight of distilled water), positive control (obese rats receiving 20 mg/kgBW of Orlistat), test groups 1, 2 and 3 all obese and receiving 250, 500, 1000 mg/kgBW of fish oil respectively by oral gavage. After 28 treatment days, oral glucose tolerance test was performed, fasting blood glucose, lipid profile (total cholesterol, triglycerides, high density lipoproteins, low density lipoproteins and very low density lipoproteins) and serum markers (aspartate aminotransferase, alanine transferase, lactate dehydrogenase and creatinine) were measured using commercial kits. The results from S. maderensis oil quality indices revealed that the FFA content (0.98 ± 0.27%), IV (118.41 ± 5.83 gI2/100g), PV (7.94 ± 0.39 meqO2/kg), P-AV (18.57 ± 0.30) and TOTOX (34.24 ± 0.80) were all within the codex alimentarius commission recommended ranges meaning that oil may be safe for consumption. The fish oil significantly (p<0.05) decreased body weight, food intake and serum lipids (TC, TG and LDL-c) but increased HDL-c. Serum AST, ALT and insulin levels were significantly decreased (p<0.05). HOMA-IR, atherogenic index of plasma and AST/ALT ratio were all significantly decreased (p<0.05) in treated groups. All these results suggested that the oil showed a protective effect against cardiometabolic risks. This data may be useful for the design a preventive plan against obesity and cardiometabolic risks.
Keywords
Cardiometabolic risks, Obesity, Cardiometabolic dysfunction, Insulin resistance, Diabetes mellitus Type-2, Cholesterol
Introduction
Numerous evidences have shown that sustained consumption of High-saturated Fat Diet (HFD) can result in obesity, diabetes mellitus type-2 and as well as other related diseases like cardiovascular diseases and cancer [1]. Cardiovascular Diseases (CVD) are on the rise in Sub-Saharan Africa, and a large proportion of the adult population is thought to sufer from at least one cardiometabolic risk factor [2]. In forty years, the prevalence of obesity has doubled and one third of the world ‘s population (approximately 39%) is overweight or obese [3]. In 2020, up to 39 million children under the age of 5 were overweight or obese. There is an increased risk of cardiovascular disease associated with obesity and about 90% of obese people have diabetes mellitus type-2 [4]. The hypertriglyceridemia, one of the obesity phenotypes, is becoming a risk factor for many chronic diseases [5]. Obesity is a public health problem worldwide that contributes to the development of many metabolic alterations such as hepatic steatosis, glucose intolerance and hypertension [6]. These metabolic derangements lead to the development of Type-2 Diabetes Mellitus (T2DM), non-alcoholic fatty liver disease and cardiovascular diseases, which are the main causes of morbidity and mortality in many countries [7]. Cardiovascular Diseases (CVDs) are the leading causes of death globally, accounted for 17.7 million deaths in 2016, and are mostly prevalent in low-to middle-income countries 2 [8]. Cardiovascular Diseases (CVDs), such as ischemic heart disease and stroke, are major causes of death worldwide [9]. Risk factors for CVDs include dyslipidemia, obesity, increased blood pressure and diabetes mellitus [10]. Diabetes grows worse over time and comes with major complications such as hyperlipidemia, and Cardiovascular Diseases (CVDs) [11]. According to statistical data of the International Diabetes Federation, more than 450 million people suffered from diabetes worldwide in 2017, and this number was expected to hit 629 million by 2045 [12]. Lifestyle changes which focus on proper nutrition and physical activity constitute the primary approach for treating obesity [13]. Fish oil rich in omega-3 polyunsaturated fatty acids such as eicosapentaenoic acid and docosahexaenoic acid have been reported to reduce obesity [14-16] prevent cardiovascular disease risk [17- 19] as well as overcome the insulin resistance [14,20]. Sardinella maderensis is a sea fish commonly consumed in Cameroon but little information is available on its oil. Therefore, the aim of this study was to evaluate the effects of S. maderensis oil based Omega-3 PUFA supplementation on obesity, insulin resistance and cardiometabolic dyfunction in obese albino rats. Organisation of the template.
A template (with its file name ending on .dot, rather than on .doc) in Word® is a “mold” that formats documents based on it. If you click ‘New’ on the ‘File’ menu, what you see and open are in fact templates. To use the Computer Physics Communications template, you should first save it with the other templates, probably in a directory called ‘…\Microsoft Office\Templates’. If you cannot find it, go to the ‘Tools’ menu, choose ‘Options…’, click the ‘File locations’ tab and see which directory is specified for ‘User templates’. After saving the template in that directory, you can start using it via ‘New’ on the ‘File’ menu.
The template formats your text by using a Word® feature called ‘Styles’. Styles define the format (or appearance) of a paragraph of text as regards letter size, indentation, line spacing, etc. If you’re not familiar with using styles, do not worry, the template arranges everything for you in a user-friendly way.
Materials and Methods
Sample collection
Twenty-five kilograms of sardine with weight range from 106-207 grams and length range from 19-21 cm were purchased from a cold store in Buea, South-west region at arrival as shown in Figure 1. Immediately after purchase, they were placed in ice with a fish/ice ratio of 1:2 (w/w) and transported to the Life Science Teaching Laboratory of the University of Buea and kept in the freezer for future use.
Sample preparation: Prior to be used, the fish were thawed, the internal organs removed and the fish were washed to remove the residual blood. Bones, scales and fins were removed with the help of a sharp knife. Fish fillet were obtained by cutting the fish lengthwise along the backbone to obtain as much flesh as possible.
Oil extraction: The oil was extracted by the Bligh and Dyer method [21]. All the fillets were ground and mixed with water, chloroform, and methanol in solvent ratios 1:1:4 (ml/ml/ml) respectively. The mixture was then blended, squeezed using a clean linen cloth, and filtered using a coffee filter paper number 2, within 10 to 15 minutes. The polar phase, water and methanol were removed using a separating funnel. Chloroform (lower liquid layer) was collected and filtered using a coffee filter paper number 2 to remove moisture and impurities. Oil was obtained by evaporating the solvent at 40oC using a vacuum rotary evaporator.
Oil quality indices: Percentage free fatty acids
Colorimetric method principle: The free fatty acids in the sample, under conditions of pH<7.0 react with a chromagen to develop a colour whose optical density is proportional to the concentration of acidity of the fat, expressed as percentage of oleic acid.
Into a 250 millilitres conical flask, 2 grams of oil were weighed and added, 100 ml of 95% ethanol previously neutralized with 2 ml of phenolphthalein indicator were also added. The conical flask was placed on a hot water bath until the oil was completely dissolved in the solvent. The hot solution was titrated with 0.1 mole KOH until a pink color appeared [22].
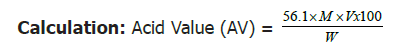
Where V=Titre value
M=Molarity of KOH used
W=Weight in grams, of sample
This acid value was then converted to percentage free fatty acids (%FFA) using the following formula [22]:
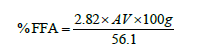
Iodine value
Principle: Fatty acids react with a halogen (iodine) resulting in the addition of the halogen at the C=C double bond site.
Colorimetric method: 0.1 g of oil was weighed into a 500 ml conical flask with glass stopper, to which 25 ml of chloroform was added. Approximately 25 ml of Wij’s solution was pipetted and the flasks were kept in dark for half an hour. A blank was carried out simultaneously in the same manner as test sample but without oil. Then, 15 ml of potassium iodide solution was added. The liberated iodine was titrated with standardized sodium thiosulphate solution, using starch as indicator at the end until the blue colour formed disappeared after thorough shaking with the stopper [22].
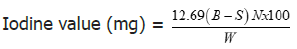
Where, B =Volume in ml of standard sodium thiosulphate solution required for the blank.
S=Volume in ml of standard sodium thiosulphate solution required for the sample.
N=Normality of the standard sodium thiosulphate solution.
W=Weight in g of the sample.
Peroxide value
Principle: It is based on peroxides liberating iodine from potassium iodide. That is, the amount of peroxides is calculated back by the amount of sodium thiosulfate consumed.
The peroxide value was determined by AOCS official method. The test oil sample was filtered through a Whatman filter paper to remove moisture and impurities. A 0.5 g sample of filtered oil was accurately weighed to 0.05 g in a 250 ml Erlenmeyer flask. 30 ml of 3:2 acetic acid chloroform was added and swirled to mix well. Two blank samples were simultaneously prepared without the addition of fish oil. To the samples, 0.5 ml of saturated potassium iodide solution was added and allowed to stand for exactly 1 min. Saturated potassium iodide solution was prepared by adding 10 g potassium iodide to 6 ml boiled distilled water. After the standing time 30 ml of distilled water was immediately added to the oil samples and swirled to mix. The samples ware titrated against 0.1 N sodium thiosulfate until the yellow iodine color disappeared. Starch indicator (2 ml) was added and the titration was continued against 0.1 N sodium thiosulfate until the blue color disappeared.
Calculation:
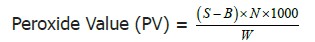
Where:B =Volume of titrant, ml of blank
S =Volume of titrant, mL of sample
N =Normality of sodium thiosulfate solution
W =Weight of sample, (g)
P-anisidine value
Principle: Based on the fact that aldehydes derived from primary oxidation products such as peroxides will react with p-anisidine.
P-anisidine value was determined by AOCS official method. A 0.5-4 ± 0.001 g sample of oil was accurately weighed in a 25 ml volumetric flask. The oil samples were then dissolved and diluted with 25 ml iso-octane. The Absorbance (AB) of the oil sample was measured at 350 nm using a spectrophotometer. A 5 ml sample of oil was pipetted into one test tube and 1 ml of p-anisidine reagent will be added. Then, 5 ml of iso-octane was added to another test tube with 1 ml of p-anisidine reagent and used as a blank. The p-anisidine reagent was prepared by adding 0.25 g p-anisidine to 100 ml of glacial acetic acid. After 10 minutes, the Absorbance (AS) of the oil sample with the p-anisidine reagent was measured immediately at 350 nm using a spectrophotometer.
Calculation:
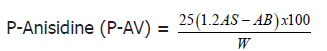
Where: AS=Absorbance of the fat solution after reaction with the p-anisidine reagent
AB=Absorbance of the fat solution
W=Weight of oil (g)
TOTOX value
The Totox value (Total Oxidation Value) will be obtained from the peroxide and the anisidine value by calculation.
TOTOX = 2PV + p − AV
Animal bioassay
Ethical consideration: An ethical clearance for animal handling and care was obtained from the University of Buea-Institutional Animal Care and Use Committee (UB-IACUC) with permit number UB-IACUC N0 03/2023 in order to carry out this study.
Preparation of animal feed and formulation of the obese rat diet: Normal diet 600 grams of maize flour, 200 grams of soya beans, 190 grams fish powder and 10 grams of salt for every kilogram of food.
High Fat Diet (HFD), 600 grams of normal diet mixture, plus 290 grams of lard (saturated fat from pig), and 110 grams of boiled egg yolk for cholesterol as reported by Othman et al. [23]. After a dough-like consistency formed using water, feed were shaped into small hand-balls and kept at 40°C overnight to feed the rats in the next morning.
Induction of obesity, animal grouping and feeding: Male albino rats aged 3-4 months, weighting 150- 200 grams was purchased at the animal house of the department of animal biology and conservation of the university of Buea, kept in the animal house of the faculty of science. The rats were allowed to acclimatize for a week in caged baths (containing sawdust) with water and food given ad-libitum before the commencement of the experiment. During the experimental phase, a total of 36 adult albino rats were randomly and evenly distributed into six groups (numbered 1-6) of six animals each. High fat diet was fed to groups 2,3,4,5 and 6 for 60 days prior to treatment period.
Rats with Lee index greater than or equal to 310 were considered obese Figure 2 and oil treatment was done for 28 days afterwards. The growth response, daily food and water intake of each group was calculated for the entire duration of the experiment.
Normal group: Healthy rats fed with normal rodent chow.
Negative control: Obese+distilled water (250 mg/ kgBW).
Positive control group: Obese rats with oral administration of 20 mg per kg body weight orlistat Figure 3 for the last 28 days consecutively, 1/3 of that used by Bukhari et al. [24].
Test groups 1, 2 and 3,Obesity+250, 500, 1000 mg/kg body weight of Sardinella maderensis oil respectively by oral gavaging according to Keapai et al. using a curved 18 gauge, 2.25 ball diameter and 2 inches in length [25].
After 28 treatment days (at the end of the experiment), the rats were fasted overnight before being anesthetized with both ketamine (60 mg/kg) and xylazine (10 mg/kg), then whole blood was collected from the rats by cardiac puncture using 5 ml syringes [26].
Biochemical analysis
In order to evaluate the effect fish oil on the rats, the following tests were conducted:
Oral glucose tolerance test: The Oral Glucose Tolerance Test (OGTT) was used to test insulin resistance, according to the tests used in a previous study by de Castro Moreira et al. [27]. After the rats fasted for 12 h, 2 g/kg body weight of glucose solution were administered by oral gavage to each rat. The blood glucose was collected from a tail vein using a glucose meter according to the user instruction’s guideline (ACCU-CHEK per-forma, Roche Diagnistics GmbH, Mannheim, Germany) at 0, 30, 60, 120 and 240 min, and the data expressed as an Area Under the Curve (AUC).
Fasting blood glucose and insulin measurement: Fasting blood glucose was measured at the end of the experimental phase. Serum was collected from the rats after centrifuging at 3000 rpm for 20 min. The serum insulin level was measured using a Fluorecare kit (Microprofit Biotech, Shenwhen, China) according to the manufacturer’s instructions. The insulin resistance index of Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated according to the following formula Salgado et al.[28]
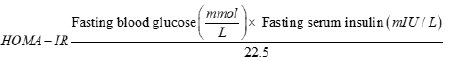
Measurement of serum enzymes and lipid profile: Blood was placed into a sterile Vacutainer plastic tube (BD Vacutainer, Plymouth, UK). Serum was separated by centrifugation (3000 rpm, 20 min) and transferred to Eppendorf tubes. All serum samples were stored at -80°C until analysis. Lipid profile (Triglycerides, total cholesterol, LDL-cholesterol, HDL-cholesterol, VLDL-cholesterol), and serum markers (aspartate transferase, alanine transferase, lactate dehydrogenase, creatinine) were measured with commercial kits (Chronolab).
Atherogenic index of plasma
Using results from the lipid profile tests for each group, the athrogenic index of plasma was calculated according to Dobiášová, et al. [29] using the formula:
AIP = Log10(TG / HDL − c)
AST/ALT ratio: Using results from the lipid profile tests for each group, the AST/ALT ratio was calculated according to de Ritis et al.[30].
Statistical analysis: Data was analyzed using Graph- Pad Prism version 8 (GraphPad Software, Inc, La Jolla, USA) and was expressed as mean ± SD and analyzed by one-way ANOVA analysis of variance with Bonferroni test. The blood glucose Area Under the Curve (AUC) was calculated using excel 2013 with the formula AUC=(B1+B2)/2*(A2-A1). The results were considered as statistically significant when the P value was less than 0.05.
Result and Discussion
Oil quality indices
In order to know the safety of the Sardinella maderensis oil for consumption, the oil quality indices were analyzed and presented on the Table 1.
| Indices | Value | Codex safety level |
|---|---|---|
| Percentage free fatty acid (%) | 0.98 ± 0.27 | ≤ 2.5 |
| Iodine value (gI2/100 g) | 118.41 ± 5.83 | |
| Peroxide value (meqO2/kg) | 7.94 ± 0.39 | ≤ 10 |
| P-anisidine value | 18.57 ± 0.30 | ≤ 20 |
| Total oxidation value | 34.24 ± 0.80 | ≤ 40 |
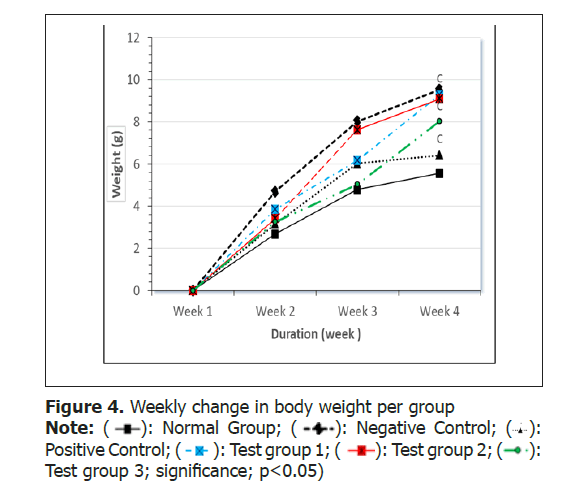
Effect of sardinella maderensis on rats: Body weight and food intake
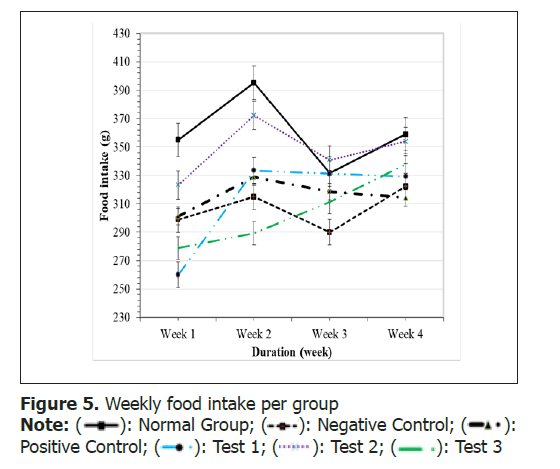
In an effort to explore the improvement in obesity, the change in body weight Figure 4 and the rats’ food intake were recorded weekly Figure 5.
The food intake from per week significantly increased (p>0.05) from week 1 to week 2 in all high fat diet fed groups when compared to normal group.
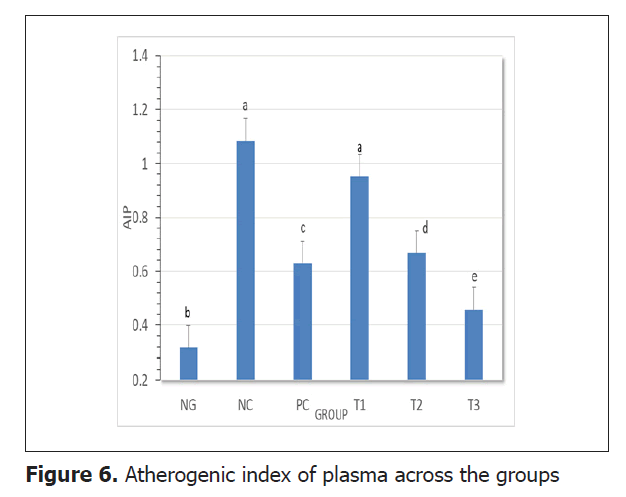
Serum lipid profile: To investigate the effect of fish oil supplementation on the predisposition to obesity, cardiovascular diseases and diabetes mellitus type 2, the serum lipid profile (TC, TG, HDL-c, LDL-c and VLDL-c) was assessed and presented on Table 2. The negative control group showed a significant increase (p<0.05) in TC, TG, LDL-c and VLDL-c but decreased HDL-c levels when compared to the normal group. The positive control, test groups 1, 2 and 3 all showed a significant decrease (p<0.05) in serum TC, TG, LDL-c VLDL-c but increased HDL-c levels when compared to the negative control group. The highest dose of fish oil (1000 mg/kg BW) appeared to be more effective. To assess the risk of atherosclerosis, the Atherogenic Index of Plasma (AIP) was calculated and the values displayed on Figure 6.
| Groups Tests | NG | NC | PC | T1 | T2 | T3 |
|---|---|---|---|---|---|---|
| TC | 1888.88 | 2200.00 | 1766.66 | 1948.14 | 1788.88 | 1600.00 |
| (mg/dl) | ± 0.23b | ± 157.13a | ± 47.14c | ± 26.36d | ± 141.42e | ± 0.09f |
| TG | 2450 | 3316.66 | 2333.33 | 3366.67 | 2866.67 | 2766.67 |
| (mg/dl) | ± 212.13b | ± 259.27a | ± 0.06c | ± 188.56a | ± 77.12d | ± 94.28e |
| LDL-c | 191.53 | 1263.67 | 752.91 | 823.08 | 817.37 | 96.82 |
| (mg/dl) | ± 0.12b | ± 135.11a | ±18.42c | ± 41.29d | ± 0.20e | ± 0.03f |
| VLDL-c | 221.53 | 1263.67 | 752.87 | 893.51 | 586.75 | 83.48 |
| (mg/dl) | ± 42.42b | ± 0.90a | ± 0.05c | ± 70.71d | ± 75.42e | ± 18.85f |
| HDL-c | 1177.35 | 272.98 | 547.12 | 381.3 | 628.8 | 963.17 |
| (mg/dl) | ± 0.50b | ± 29.83a | ± 28.77c | ± 88.35d | ± 14.72e | ± 0.04f |
Note: NG: Normal Group; NC: Negative Control; PC: Positive Control; T1: Test group 1; T2: Test group 2; T3: Test group 3; Significance; p<0.05
There was a statistically significant decrease (p<0.05) in AIP in test groups 1, 2 and 3 when compared to the negative control group.
Assay of serum enzyme markers
As a means to inspect the effects of fish oil supplementation on cardiometabolic dysfunction, the level of serum enzyme markers (LDH, AST, ALT) and creatinine were assessed and shown in Table 3.
| Groups | NG | NC | PC | T1 | T2 | T3 |
|---|---|---|---|---|---|---|
| Tests | ||||||
| ALT | 12.54 | 34.88 | 14.15 | 25.95 | 21.58 | 16.91 |
| (µ/L) | ± 2.06b | ± 5.69a | ± 1.34c | ± 3.71d | ± 4.94d | ± 0.03c |
| AST | 27.67 | 52.21 | 29.75 | 40.83 | 42.00 | 32.95 |
| (µ/L) | ± 8.24b | ± 11.96a | ± 2.6673b | ± 3.29c | ± 6.59d | ± 2.88e |
| Creatinine | 1.28 | 1.61 | 1.22 | 1.111 | 0.77 | 0.77 |
| (mg/dl) | ± 0.08a | ± 0.39a | ± 0.47a | ± 0.68a | ± 0.15b | ± 0.15c |
| LDH | 312.74 | 469.52 | 375.94 | 488.39 | 424.37 | 467.05 |
| (µ/L) | ± 68.49b | ± 21.53a | ± 18.57c | ± 5.80a | ± 56.88a | ± 5.80a |
In this study, all markers in three test groups showed significant decline (p<0.05) except for LDH and creatinine when compared to negative control.
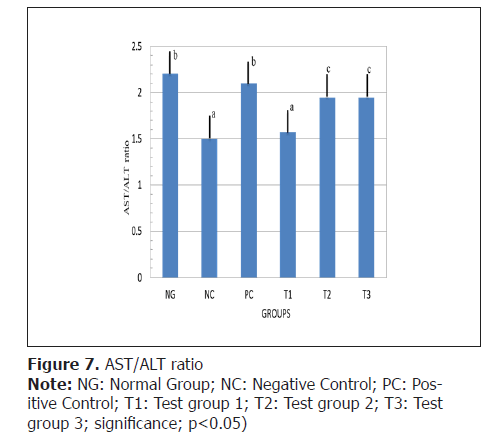
AST/ALT ratio: To determine the risk of cardiovascular diseases, the AST/ALT ratio was obtained and presented on Figure 7.
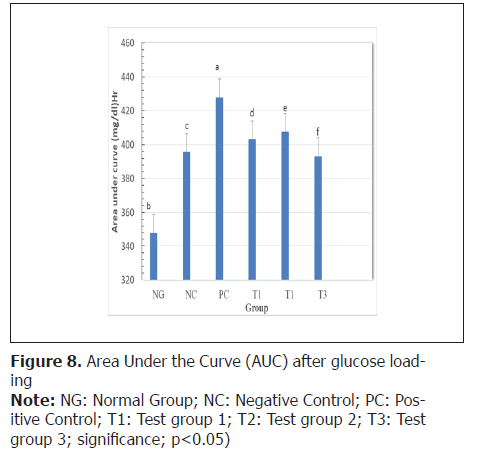
Oral glucose tolerance: In order to assess impaired glucose tolerance, the oral glucose tolerance test was conducted and the Area Under the Curve (AUC) was represented on Figure 8. The oral glucose loading induced significant increase of blood glucose level in rats of all groups. At the end of the test, curves were plotted for each group and the decrease between the area under each curve between the 3 test groups and the positive control group was statistically significant (p<0.05).
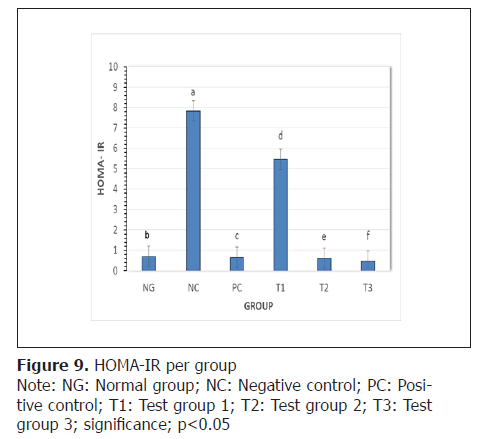
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR): In an effort to scrutinize the amount of insulin needed to keep the blood sugar in check, Homeostatic Model Assessment for Insulin Resistance (HOMA- IR) was calculated from values of table 3 and the results represented on Figure 9. The decrease in HOMA-IR in the test groups was statistically significant (p<0.05) when compared to negative control.
In order to study the safety of Sardinella maderensis oil for consumption, the oil quality indices where evaluated. The oil contains 0.98 ± 0.27% of Free Fatty Acid (FFA); this value of FFA was within the safety range of ≤ 2.5% recommended by the Codex alimentarius commission. This result was similarly to the FFA content (0.92 ± 0.08%) obtained in the study of Aloo [31] for Oreochromis niloticus (tilapia) viscera oil unlike that of Cyprinos carpio (carp fish) (3.35 ± 0.02%) reported by Crexi et al. [32]. Also, FFA content was employed to assess fish deterioration during frozen storage de Koning et al. [33] suggesting that S. maderensis oil may have a long shelf life.
The iodine value indicates the number of reactive double bonds present in an oil. Ideally, this index is not a quality indicator but rather a composition indicator Miller [34]. A higher iodine value indicates more double bonds (unsaturation) inferring a high caution in terms of measures to reduce or slow down autoxidation. The iodine value of the S. maderensis oil was 118.41 ± 5.83 gI2/100 g and according to Mwangi et al. [35] the iodine value of fish oil can range up to 185 indicating a high level of double bonds (unsaturation).
The peroxide value obtained (7.94 ± 0.39 meq O2/kg) was within the recommended range of ≤ 10 mill equivalent of active oxygen/kg of oil by the Codex Alimentarius Committee for all edible oils [36]. This value is similar to that of other edible oils: Farmed salmon, seal, cod liver and wild salmon oil were 9.17, 7.04, 6.92 and 5.13 meqO2/kg oil, respectively Dave et al. [37] indicating a low level of primary oxidation products characterized by the presence of hydroperoxides in oil [37].
The P-anisidine value (18.57 ± 0.30) was within the recommended range of safety by the Codex Alimentarius Commission (P-anisidine value ≤ 20). Similarly, the p-Anisidine values of farmed salmon, seal, cod liver and wild salmon oil were 3.36, 4.21, 6.20 and 9.67, respectively Dave et al. [37]. This P-Anisidine value suggests that S. madernsis oil may contain low secondary oxidation products such as aldehydes of α-and β-unsaturation [38].
The total oxidation value (34.24 ± 0.8) was within the recommended codex alimentarus commission safety range (TOTOX ≤ 40) for edible oils, indicating high primary (hydroperoxides) and secondary (aldehydes) oxidative stability for storage [36].These oil quality indices suggested that S. maderensis oil is good for consumption but it must not be kept exposed at room temperature. This fish oil was then administered to rats to see its effects on cardiometabolic risks.
Rats fed HFD with Sardinella maderensis oil supplementation showed a significant decrease (p<0.05) in body weight in comparison to the negative control group, but were still greater in weight than those of the normal control group. On the other hand, food intake significantly increased from week 1 to 4 in HFD fed groups when compared to the normal group. These results are similar to the study done by Godea et al.[39] who reported that fish oil supplementation significantly reduced (p<0.05) body weight and proposed that the hypolipidemic and antioxidant activities of fish oil are accountable for its favorable benefits on body weight gain. This means that the fish oil promoted lipid oxidation and excretion therefore leading to lose of weight.
Concerning lipid profile, HFD consumption manifested a significant (p<0.05) elevation in serum TC, TG, HDL-c, LDL-c, and VLDL-c in rats when compared to rats receiving the normal control diet. Similarly, it has been shown by other investigators that the levels of TC, TG, LDL-c and VLDL-c were substantially elevated in HFD rats in comparison to normal controls [40,41].
Atherogenic Index of Plasma (AIP) is an important predictor of cardiovascular disease (atherosclerosis) [42]. Many studies have reported that elevated AIP values are positively correlated with diabetes [43,44] and obesity indicators [42,45]. AIP of plasma was significantly decreased (p<0.05) in the three test groups indicating a lower risk of obesity, CVDs and T2DM as compared to the negative control. It was equally noticed in other experiments that lower values of AIP turned to be healthier [42,45]. Although S. maderensis oil caused a significant reduction (p<0.05) in these parameters, they were still statistically higher than those of the normal control group. This implies that the S. maderensis oil has an ameliorative effect on the alterations of a lipid profile that occurs due to HFD consumption. These results are important for showing that S. maderensis oil significantly reduced (P<0.05) TC, TG, HDL-c, LDL-c, and VLDL-c which is similarly to the study conducted by Haimeur et al., Godea et al [39,46]. Likewise, in this investigation there was a significant increase (p<0.05) of HDL-c level in serum observed by the effect of supplementation of S. maderensis oil in comparison with that fed on HFD and control diet. This is because fish oil raised lipoprotein lipase activity to produce more HDL-c which helped remove other forms of cholesterol from the bloodstream. Our findings were similar to those of Albracht et al. [47] but different from those of Han et al. in which there was no significant change (P>0.05) in HDL-c levels when compared to negative control rats [41]. The beneficial effect on serum lipid profile may be attributed to the fact that S. maderensis oil probably contains omega-3 fatty acids, which contribute to lipid excretion via bile, depleting the hepatic cholesterol pool and enhancing free cholesterol synthesis [41]. Hence, omega-3 PUFAs, as mainly Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) are substrates for signaling molecules regulating the liver lipid metabolism Krebs et al., Bowen et al. [48,49]. This feature is achieved through (i) Transcriptional activation of FA oxidation by Peroxisome Proliferator-Activated Receptor alpha (PPAR-α); and (ii) Suppression of de novo lipogenesis via down-regulation of Sterol Regulatory Element-Binding Protein 1 (SREBP- 1c) Lombardo et al., Hernandez et al. [50,51]
Regarding serum enzyme markers, there was a significant decrease (p<0.05) in AST and ALT levels but no significant change (p>0.05) was observed in creatinine and LDH. Our findings in AST and ALT levels were in line with findings from other studies Aslam et al., Abdul-Lattif, Galal, et al.[52-54]. The release of ALT and AST from liver cells into the bloodstream represents hepatocellular damage. The absence of statistical difference between the groups can mean that there was not enough hepatocellular damage to change the levels of liver markers as seen in LDH and creatinine [55,56].
Current findings emphasized that a higher AST/ALT ratio can be a marker for cardiovascular diseases. Similarly, Yokoyama et al. [57] revealed that the elevated AST/ALT ratios predict all-cause mortality and cardiovascular mortality but this effect was significantly alleviated by the fish oil. In terms of the results of the present study, the possible explanations were as follows: In the first place, a high AST/ALT ratio was associated with an increase in non-alcoholic fatty liver disease, which could cause dyslipidemia. Second, from previous studies the AST/ ALT ratio was an indicator of insulin resistance Simental- Mendia et al., Zhao et al. [58,59] and insulin resistance was closely related to Peripheral Artery Disease (PAD) Britton et al., Pande et al.[60,61]. Therefore, we suspect that the increase in AST/ALT ratio might cause insulin resistance and dyslipidemia, further leading to increased risk for cardiovascular diseases as reported by Zoppini et al., Feng et al.[62,63].
High fat diet fed rats manifested substantial upraise in insulin levels and HOMA-IR as compared to negative control rats and these results were in line with results from Bashir et al., Hou et al. [64,65] who used fish oil. We observed that S. maderensis oil with HFD significantly decreased (p<0.05) insulin levels and HOMA-IR but not fasting blood glucose when compared to the negative control. These findings were in agreement to those of a previous study by Yu et al. but had a decrease in fasting blood glucose [66]. However, this indicated that S. maderensis oil showed a significant beneficial effect on insulin resistance. Fish oil rich in long-chain omega 3 polyunsaturated fatty acids, mainly EPA and DHA, have been shown to prevent the development of insulin resistance and improve insulin sensitivity in adult human and animal studies in a dose dependent manner [67-75]. The beneficial effect of fish oil on insulin sensitivity could be explained by some potential mechanisms [76-82]. Inflammation is a critical mechanism in the pathogenesis of insulin resistance and fish oil intake has been reported to increase anti-inflammatory molecules and suppress pro-inflammatory mediators, which may in turn improved insulin sensitivity [64,69,83-89] .
This protective effect of S. maderensis oil may be due to omega-3 fatty acids such as DHA/EPA which have been reported to alleviate the metabolic disorders accompanying obesity such as hyperlipidemia, cardiac biomarkers and insulin resistance [70,71,90-92].
Conclusion
This present study aimed to assess the protective effect of Sardinellla maderensis oil on insulin resistance and biomarkers of cardiovascular function in obese albino rats. Sardinellla maderensis oil was extracted, characterized and given to rats fed with high fat diet. Then serum enzyme markers, lipids and insulin resistance were evaluated. The percentage free fatty acid, iodine value, total oxidation value, peroxide value and P-anisidine value were all within the recommended codex alimentarius commission range implying the oil is stable at room temperature and safe for consumption. Sardinella maderensis oil significantly decreased (P<0.05) body weight and serum lipids (TC-c, TG, VLDL-c and LDL-c) but increased HDL-c. AST and ALT were significantly decreased unlike creatinine and LDH which both showed no significant change (P>0.05). The serum insulin level and HOMA-IR were significantly diminished in S. maderensis oil treated groups. Implying the oil showed beneficial effect in treating obesity and cardiometabolic dysfunction. This data may be useful to design a preventive plan for cardiometabolic risks.
Compliance of ethical standards
An ethical clearance for animal handling and care was obtained from the University of Buea-Institutional Animal Care and Use Committee (UB-IACUC) with permit number UBIACUC N 0 03/2023 in order to carry out this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Declaration of competing interest
All authors declared no conflict of interest.
References
- Denver P, Gault VA, Mcclean PL. Sustained high‐fat diet modulates inflammation, insulin signalling and cognition in mice and a modified xenin peptide ameliorates neuropathology in a chronic high‐fat model. Diabetes Obes Metab. 2018;20(5):1166-1175.
- Dwyer-Lindgren L, Cork MA, Sligar A, Steuben KM, Wilson KF, Provost NR, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570(7760):189-193.
- Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10.
[Crossref] [Google Scholar] [Indexed]
- Cardiovascular Diseases (CVDs). World Health Organis. 2021.
- Liu S, Chang X, Yu J, Xu W. Cerasus humilis cherry polyphenol reduces high-fat diet-induced obesity in C57BL/6 mice by mitigating fat deposition, inflammation, and oxidation. J Agric Food Chem. 2020;68(15):4424-4436.
- Engin A. The definition and prevalence of obesity and metabolic syndrome. Obesity Lipotoxic. 2017:1-7.
[Crossref] [Google Scholar] [Indexed]
- Guglielmi V, Sbraccia P. Obesity phenotypes: Depot-differences in adipose tissue and their clinical implications. Eat Weight Disord. 2018;23(1):3-14.
[Crossref] [Google Scholar] [Indexed]
- Non communicable diseases. World Health Organis. 2023.
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1-25.
[Crossref] [Google Scholar] [Indexed]
- Joseph P, Leong D, Mckee M, Anand SS, Schwalm JD, Teo K, et al. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ Res. 2017;121(6):677-694.
- González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJ, et al. Obesity (Primer). Nat Rev Dis. 2017;3(1).
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281.
- Rodríguez JE, Campbell KM. Past, present, and future of pharmacologic therapy in obesity. Prim Care. 2016 ;43(1):61-67.
- Adamcova K, Horakova O, Bardova K, Janovska P, Brezinova M, Kuda O, et al. Reduced number of adipose lineage and endothelial cells in epididymal fat in response to omega-3 PUFA in mice fed high-fat diet. Mar Drugs. 2018;16(12):515.
- Chiu CY, Wang LP, Liu SH, Chiang MT. Fish oil supplementation alleviates the altered lipid homeostasis in blood, liver, and adipose tissues in high-fat diet-fed rats. J Agric Food Chem. 2018;66(16):4118-28.
- Albracht-Schulte K, Gonzalez S, Jackson A, Wilson S, Ramalingam L, Kalupahana NS, et al. Eicosapentaenoic acid improves hepatic metabolism and reduces inflammation independent of obesity in high-fat-fed mice and in HepG2 cells. Nutrients. 2019;11(3):599.
[Crossref] [Google Scholar] [Indexed]
- Arca M, Borghi C, Pontremoli R, de Ferrari GM, Colivicchi F, Desideri G, et al. Hypertriglyceridemia and omega-3 fatty acids: Their often overlooked role in cardiovascular disease prevention. Nutr Metab Cardiovasc Dis. 2018;28(3):197-205.
[Crossref] [Google Scholar] [Indexed]
- Hu Y, Hu FB, Manson JE. Marine omega‐3 supplementation and cardiovascular disease: An updated meta‐analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8(19): e013543.
[Crossref] [Google Scholar] [Indexed]
- Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020;40(5):1135-1147.
[Crossref] [Google Scholar] [Indexed]
- Lepretti M, Martucciello S, Burgos Aceves MA, Putti R, Lionetti L. Omega-3 fatty acids and insulin resistance: Focus on the regulation of mitochondria and endoplasmic reticulum stress. Nutrients. 2018;10(3):350.
- Cunniff PA. Official methods of analysis of AOAC international. AOAC Intern. 1999.
- AOAC International, Horwitz W, Latimer GW. Official methods of analysis of AOAC international. AOAC Intern. 2005.
- Othman ZA, Wan Ghazali WS, Noordin L, Mohd. Yusof NA, Mohamed M. Phenolic compounds and the anti-atherogenic effect of bee bread in high-fat diet-induced obese rats. Antioxidants. 2019;9(1):33.
[Crossref] [Google Scholar] [Indexed]
- Bukhari HM, Zahran SE, Bakr ES, Sahibzadah FA, Header EA. Comparison study between drugs (orlistat and chitocal) and food supplements (green tea and apple cider vinegar) for weight loss and hepatoprotection in rats. Egyptian J Hosp Med. 2021;83(1):1218-1223.
- Keapai W, Apichai S, Amornlerdpison D, Lailerd N. Evaluation of fish oil-rich in MUFAs for anti-diabetic and anti-inflammation potential in experimental type 2 diabetic rats. Korean J Physiol Pharmacol. 2016;20(6):581.
[Crossref] [Google Scholar] [Indexed]
- Ahmed AZ, Mumbrekar KD, Satyam SM, Shetty P, D’Souza MR, Singh VK. Chia seed oil ameliorates doxorubicin-induced cardiotoxicity in female wistar rats: An electrocardiographic, biochemical and histopathological approach. Cardiovasc Toxicol. 2021;21:533-542.
[Crossref] [Google Scholar] [Indexed]
- de Castro Moreira ME, Natal DI, Toledo RC, Ramirez NM, Ribeiro SM, dos Anjos Benjamin L, et al. Bacupari peel extracts (Garcinia brasiliensis) reduce high-fat diet-induced obesity in rats. J Funct Foods. 2017;29:143-153.
- Salgado AL, Carvalho LD, Oliveira AC, Santos VN, Vieira JG, Parise ER. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol. 2010;47:165-169.
- Dobiás̆ová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clin Biochem. 2001;34(7):583-588.
- de Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis: The transaminase serum activities. Clin Chim Acta. 1957;2(1):70-74.
[Crossref] [Google Scholar] [Indexed]
- Aloo JO. Development of refined oil from Lake Victoria Nile perch (Lates niloticus) viscera. 2014. Univ Nairobi Res Arch.
- Crexi VT, Monte ML, de Souza Soares LA, Pinto LA. Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chemistry. 2010;119(3):945-950.
- de Koning AJ, Mol TH. Quantitative quality tests for frozen fish: Soluble protein and free fatty acid content as quality criteria for hake (Merluccius capensis) stored at -18oC. J Sci Food Agric. 1991;54(3):449-458.
- Miller M. Oxidation of food grade oils. New Zealand: Plant Food Res. 2010;20101.
- Mwangi KD, Kitaka N, Otachi E. A comparative analysis of the quantity and quality of oil extracted from five commercially important freshwater fish species in Kenya. J Aquat Food Prod Techn. 2021;30(1):122-132.
[ Crossref] [Google Scholar]
- Di Caracalla VD. Standard for fish oils codex stan. FAO.2017.
- Dave D, Ramakrishnan VV, Trenholm S, Manuel H, Pohling J, Murphy W. Marine oils as potential feedstock for biodiesel production: Physicochemical characterization. J Bioprocess Biotech. 2014 ;4(5):1.
- Deepika D, Vegneshwaran VR, Julia P, Sukhinder KC, Sheila T, Heather M, et al. Investigation on oil extraction methods and its influence on omega-3 content from cultured salmon. J Food Process Techn. 2014;5(12):1-3.
- Godea S, Ciubotariu D, Danciu M, Lupușoru RV, Ghiciuc CM, Cernescu I, et al. Improvement in serum lipids and liver morphology after supplementation of the diet with fish oil is more evident under regular feeding conditions than under high-fat or mixed diets in rats. Lipids Health and Dis. 2020;19:1-8. [Crossref]
- Hussein SA, Abdel-Magid AD, Fareed FA. Biochemical effect of resveratrol on lipids profile and hepatic oxidative stress in experimentally induced obesity in female rats. Benha Vet Med J. 2017;32(1):67-74.
- Han H, Yan P, Chen L, Luo C, Gao H, Deng Q, et al. Flaxseed oil containing α-linolenic acid ester of plant sterol improved atherosclerosis in apoE deficient mice. Oxid Med Cell Longev. 2015;2015.
[Crossref] [Google Scholar] [Indexed]
- Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T, et al. Atherogenic index of plasma is a novel and better biomarker associated with obesity: A population-based cross-sectional study in China. Lipids Health Dis. 2018; 17:1-6.
[Crossref] [Google Scholar] [Indexed]
- Regmi P, Baral B, Raut M, Khanal MP. Atherogenic index of plasma for prediction of future cardiovascular disease in prediabetes and diabetes population. Atherosclerosis. 2016;252:e120.
- Scicali R, Giral P, d'Erasmo L, Cluzel P, Redheuil A, Di Pino A et al. High TG to HDL ratio plays a significant role on atherosclerosis extension in prediabetes and newly diagnosed type 2 diabetes subjects. Diabetes metab res rev. 2021;37(2): e3367.
[Crossref] [Google Scholar] [Indexed]
- Shen SW, Lu Y, Li F, Yang CJ, Feng YB, Li HW, et al. Atherogenic index of plasma is an effective index for estimating abdominal obesity. Lipids Health Dis. 2018;17:1-6.
- Haimeur A, Meskini N, Mimouni V, Ulmann L, Messaouri H, Pineau-Vincent F, et al. A comparative study on the effect of argan oil versus fish oil on risk factors for cardio-vascular disease in high-fat-fed rats. Nutrition. 2019; 57:32-39.
[Crossref] [Google Scholar] [Indexed]
- Albracht-Schulte K, Kalupahana NS, Ramalingam L, Wang S, Rahman SM, Robert-McComb J, et al. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J Nutr Biochem. 2018;58:1-6.
[Crossref] [Google Scholar] [Indexed]
- Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, et al. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes. 2006;30(10):1535-1544.
- Bowen KJ, Harris WS, Kris-Etherton PM. Omega-3 fatty acids and cardiovascular disease: Are there benefits? Curr Treat Options Cardiovasc Med. 2016;18:1-6.
[Crossref] [Google Scholar] [Indexed]
- Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J Nutr Biochem. 2006;17(1):1-3.
- Hernandez-Rodas MC, Valenzuela R, Videla LA. Relevant aspects of nutritional and dietary interventions in non-alcoholic fatty liver disease. Int J Mol Sci. 2015;16(10):25168-251698.
- Aslam F, Iqbal S, Nasir M, Anjum AA, Swan P, Sweazea K. Evaluation of white sesame seed oil on glucose control and biomarkers of hepatic, cardiac, and renal functions in male Sprague-Dawley rats with chemically induced diabetes. J Med Food. 2017;20(5):448-457.
- Abdul-Lattif RF. Effect of sesame oil on lipid profile and liver enzymes in male albino rats treated with Carbonetetrachloride (CCl4). Ibn AL-Haitham J Pure Appl Sci. 2018;23:41-48.
- Galal SM, Morsi MK, Abd El-Rahman MK, Darwish SK, Katry MA. Hepatoprotective effect of the unsaponifiable matter from olive, linseed and sesame oils against carbon tetrachloride-induced liver injury in rats. Grasas Y Aceites. 2020;71(1):e345.
- Botros M, Sikaris KA. The de ritis ratio: The test of time. Clin Biochem Rev. 2013;34(3):117-130.
[Crossref] [Google Scholar] [Indexed]
- Khadge S, Sharp JG, Thiele GM, McGuire TR, Klassen LW, Duryee MJ, et al. Dietary omega-3 and omega-6 polyunsaturated fatty acids modulate hepatic pathology. J Nutr Biochem. 2018;52:92-102.
[Crossref] [Google Scholar] [Indexed]
- Yokoyama M, Watanabe T, Otaki Y, Takahashi H, Arimoto T, Shishido T, et al. Association of the aspartate aminotransferase to alanine aminotransferase ratio with BNP level and cardiovascular mortality in the general population: The Yamagata study 10-year follow-up. Dis Markers. 2016;2016.
- Simental-Mendía LE, Rodríguez-Morán M, Gómez-Díaz R, Wacher NH, Rodríguez-Hernández H, Guerrero-Romero F. Insulin resistance is associated with elevated transaminases and low aspartate aminotransferase/alanine aminotransferase ratio in young adults with normal weight. Eur J Gastroenterol Hepatol. 2017;29(4):435-440.
[Crossref] [Google Scholar] [Indexed]
- Zhao L, Cheng J, Chen Y, Li Q, Han B, Chen Y, et al. Serum alanine aminotransferase/aspartate aminotransferase ratio is one of the best markers of insulin resistance in the Chinese population. Nutr Metab. 2017;14:1-9.
- Britton KA, Mukamal KJ, Ix JH, Siscovick DS, Newman AB, de Boer IH, et al. Insulin resistance and incident peripheral artery disease in the cardiovascular health study. Vasc Med. 2012;17(2):85-93.
[Crossref] [Google Scholar] [Indexed]
- Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of insulin resistance and inflammation with peripheral arterial disease: The national health and nutrition examination survey, 1999 to 2004. Circulation. 2008;118(1):33-41.
- Zoppini G, Cacciatori V, Negri C, Stoico V, Lippi G, Targher G, et al. The aspartate aminotransferase-to-alanine aminotransferase ratio predicts all-cause and cardiovascular mortality in patients with type 2 diabetes. Medicine. 2016;95(43):e4821.
- Feng X, Wen Y, Peng FF, Wang N, Zhan X, Wu X. Association between aminotransferase/alanine aminotransferase ratio and cardiovascular disease mortality in patients on peritoneal dialysis: A multi-center retrospective study. BMC Nephrol. 2020;21:1-9.
[Crossref] [Google Scholar] [Indexed]
- Bashir S, Sharma Y, Elahi A, Khan F. Amelioration of obesity-associated inflammation and insulin resistance in c57bl/6 mice via macrophage polarization by fish oil supplementation. J Nutr Biochem. 2016;33:82-90.
- Hou M, Zhou W, Sun L, Wang B, Shen J, Cao L, Lv H. Effect of fish oil on insulin sensitivity in children: A systematic review and meta-analysis of randomized, controlled trials. Can J Diabetes. 2021;45(6):531-538.
- Yu X, Deng Q, Tang Y, Xiao L, Liu L, Yao P, Tang H, Dong X. Flaxseed oil attenuates hepatic steatosis and insulin resistance in mice by rescuing the adaption to ER stress. J Agric Food Chem. 2018;66(41):10729-10740.
- Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J. N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab. 2002;282(3):E664-71.
- Pinel A, Morio-Liondore B, Capel F. n− 3 polyunsaturated fatty acids modulate metabolism of insulin-sensitive tissues: Implication for the prevention of type 2 diabetes. J Physiol Biochem. 2014; 70:647-658. [Crossref]
- Hernández‐Rodas MC, Valenzuela R, Echeverría F, Rincón‐Cervera MÁ, Espinosa A, Illesca P, et al. Supplementation with docosahexaenoic acid and extra virgin olive oil prevents liver steatosis induced by a high‐fat diet in mice through PPAR‐α and Nrf2 upregulation with concomitant SREBP‐1c and NF‐kB downregulation. Mol Nutr Food Res. 2017;61(12):1700479.
[Crossref] [Google Scholar] [Indexed]
- Martins AR, Crisma AR, Masi LN, Amaral CL, Marzuca-Nassr GN, Bomfim LH, et al. Attenuation of obesity and insulin resistance by fish oil supplementation is associated with improved skeletal muscle mitochondrial function in mice fed a high-fat diet. J Nutr Biochem. 2018;55:76-88.
[Crossref] [Google Scholar] [Indexed]
- Shabrina A, Tung TH, Nguyen NT, Lee HC, Wu HT, Wang W, et al. N-3 PUFA and caloric restriction diet alters lipidomic profiles in obese men with metabolic syndrome: A preliminary open study. Eur J nutr. 2020;59:3103-3112.
- Ba K, Thiaw M, Lazar N, Sarr A, Brochier T, Ndiaye I, et al. Resilience of key biological parameters of the senegalese flat sardinella to overfishing and climate change. Plos One. 2016;11(6):e0156143.
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469-84.
[Crossref] [Google Scholar] [Indexed]
- Leiter LA, Fitchett DH, Gilbert RE, Gupta M, Mancini GJ, McFarlane PA, et al. Cardiometabolic risk in Canada: A detailed analysis and position paper by the cardiometabolic risk working group. Can J Cardiol. 2011;27(2):e1-33.
- D’Agostino Sr RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care. Circulation. 2008;117(6):743-753.
[Crossref] [Google Scholar] [Indexed]
- DiNicolantonio JJ, Mccarty MF, Chatterjee S, Lavie CJ, O’Keefe JH. A higher dietary ratio of long-chain omega-3 to total omega-6 fatty acids for prevention of COX-2-dependent adenocarcinomas. Nutr Cancer. 2014;66(8):1279-1284.
[Crossref] [Google Scholar] [Indexed]
- GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13-27.
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet (London, England). 2016;388(10053):1659.
- Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(9995):743-800.
- Goff Jr DC, Lloyd-Jones DM, Bennett G, Coady S, D’agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129(25-suppl-2):S49-S73.
[Crossref] [Google Scholar] [Indexed]
- Huang XF, Xin X, Mclennan P, Storlien L. Role of fat amount and type in ameliorating diet‐induced obesity: Insights at the level of hypothalamic arcuate nucleus leptin receptor, neuropeptide Y and pro‐opiomelanocortin mRNA expression. Diabetes Obes Metab. 2004;6(1):35-44.
[Crossref] [Google Scholar] [Indexed]
- IDF diabetes atlas 7th edition. Intern Diabet Feder. 2018.
- Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54(7):585-594.
- Lee K, Yang JH. Which liver enzymes are better indicators of metabolic syndrome in adolescents: The fifth Korea national health and nutrition examination survey, 2010. Metabolic syndrome and related disorders. 2013;11(4):229-235.
- Lee MO. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am J Physiol Legacy Content. 1929;89(1):24-33.
- Leung DY, Wu X, Leung MK. A review on biodiesel production using catalyzed transesterification. Applied energy. 2010;87(4):1083-1095.
- Long MT, Pedley A, Massaro JM, Hoffmann U, Fox CS. The association between non-invasive hepatic fibrosis markers and cardiometabolic risk factors in the framingham heart study. Plos one. 2016;11(6):e0157517.
[Crossref] [Google Scholar] [Indexed]
- O'Keefe EL, Harris WS, DiNicolantonio JJ, Elagizi A, Milani RV, Lavie CJ, et al. Sea change for marine omega-3s: Randomized trials show fish oil reduces cardiovascular events. Mayo Clin Proc. 2019 ; 94(12) :2524-2533.
[Crossref] [Google Scholar] [Indexed]
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021.
[Crossref] [Google Scholar] [Indexed]
- Diabetes. World Health Organis. 2022.
- Hearts D: Diagnosis and management of type 2 diabetes. World health Organis. 2020.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88-98.
Author Info
Ghislain Mbeng NYEMB, Deffo Tiepma Ngongan Eurydice FLORE, Nkwain Armel YONGHABI, Achidi Aduni UFUAN and Bernard TIENCHEU*Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Google Scholar citation report
Citations : 2439
Clinical Nutrition and Hospital Dietetics received 2439 citations as per google scholar report
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- SCOPUS
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Web of Science
Journal Highlights
- Blood Glucose
- Dietary Supplements
- Cholesterol, Dehydration
- Digestion
- Electrolytes
- Clinical Nutrition Studies
- energy balance
- Diet quality
- Clinical Nutrition and Hospital Dietetics



 Test group 3; significance; p<0.05)
Test group 3; significance; p<0.05)